by All Things Neonatal | Jan 26, 2017 | feeding, Uncategorized, ventilation
This is becoming “all the rage” as they say. I first heard about the strategy of feeding while on CPAP from colleagues in Calgary. They had created the SINC * (Safe Individualized Feeding Competence) program to provide an approach to safely introducing feeding to those who were still requiring CPAP. As news of this approach spread a great deal of excitement ensued as one can only imagine that in these days when attainment of oral feeding is a common reason for delaying discharge, could getting an early start shorten hospital stay? I could describe what they found with the implementation of this strategy but I couldn’t do it the same justice as the presenter of the data did at a recent conference in Winnipeg. For the slide set you can find them here. As you can imagine, in this experience out of Calgary though they did indeed find that wonderful accomplishment of shorter hospital stays in the SINC group. We have been so impressed with the results and the sensibility of it all that we in fact have embraced the concept and introduced it here in both of our units. The protocol for providing this approach is the following.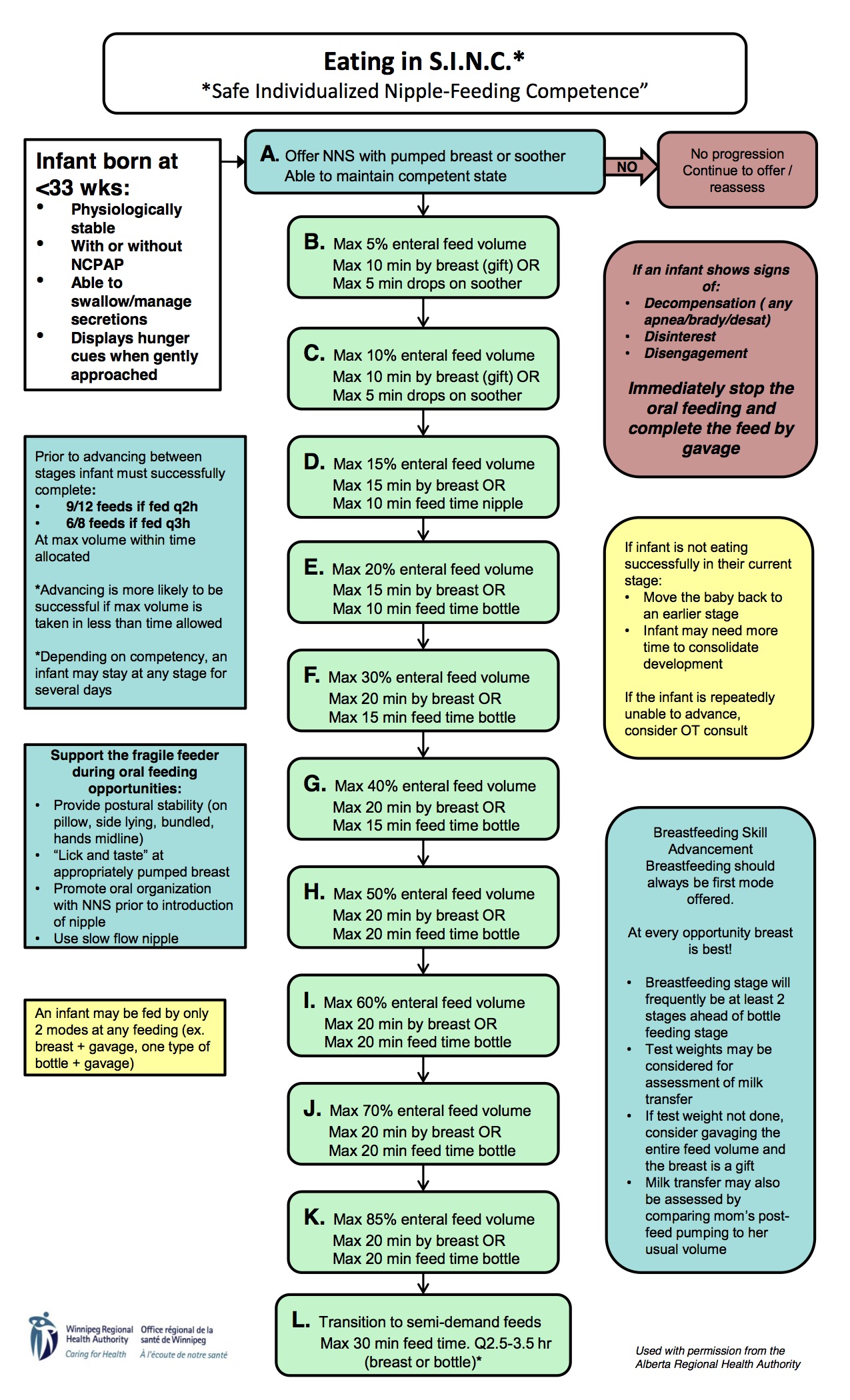
I have to admit, while I have only experienced this approach for a short time the results do seem to be impressive. Although anecdotal a parent even commented the other day that she felt that SINC was instrumental in getting her baby’s feeding going! With all this excitement around this technique I was thrown a little off kilter when a paper came out suggesting we should put a full stop to feeding on CPAP!
What caused my spirits to dampen? This study enrolled preterm infants who were still on CPAP at ≥ 34 weeks PMA and were taking over 50% of required feeding volumes by NG feeding. The goal was to look at 15 patients who were being fed on CPAP +5 and with a mean FiO2 of 25% (21-37%) using video fluoroscopic swallowing studies to determine whether such patients aspirate when being fed. The researchers became concerned when each of the first seven patients demonstrated abnormalities of swallowing function indicating varying degrees of aspiration. As such they took each patient off CPAP in the radiology suite and replaced it with 1 l/min NP to achieve acceptable oxygen saturations and repeated the study again. The results of the two swallow studies showed remarkable differences in risk to the patient and as such the recruitment of further patients was stopped due to concerns of safety and a firm recommendation of avoiding feeding while on CPAP was made.
| Table 2. Percentage of all swallows identified with swallowing dysfunction |
|
|
on-nCPAP |
off-nCPAP |
|
| Variable Mean ± s.d. |
Mean ± s.d. |
Median (q1–q3) |
Mean ± s.d. |
Median (q1–q3) |
P-value |
| Mild pen. % |
20.1±16 |
20 (4.5–35) |
15.4± 7.6 |
20 (9–20) |
0.656 |
| Deep pen. % |
43.7±15.4 |
38.5 (30–59) |
25.3± 8.8 |
25 (18.2–32) |
0.031 |
| Aspiration % |
33.5±9.4 |
30 (27.3–44.4) |
14.6± 7 |
15 (9.1–20) |
0.016 |
| Nasopharyngeal reflux % |
42.8±48.5 |
18.2 (0–100) |
44.2± 45.4 |
18.2 (5–92) |
0.875 |
Taking these results at face value it would seem that we should put an abrupt halt to feeding while on CPAP but as the saying goes the devil is in the details…
CPAP Using Ram Cannulae
Let me start off by saying that I don’t have any particular fight to pick with the RAM cannulae. They serve a purpose and that is they allow CPAP to be delivered with a very simple set of prongs and avoid the hats, straps and such of more traditional CPAP devices. We have used them as temporary CPAP delivery when moving a patient from one area to another. As the authors state the prongs are sized in order to ensure the presence of a leak. This has to do with the need to provide a way for the patient to exhale when nasal breathing. Prongs that are too loose have a large leak and may not deliver adequate pressure while those that are too tight may inadvertently deliver high pressure and therefore impose significant work of breathing on the patient. Even with appropriate sizing these prongs do not allow one to exhale against a low pressure or flow as is seen with the “fluidic flip” employed with the infant flow interface. With the fluidic flip, exhalation occurs against very little resistance thereby reducing work of breathing which is not present with the use of the RAM cannula.
A comparison of the often used “bubble CPAP” to a variable flow device also showed lower work of breathing when variable flow is used.
The Bottom Line
Trying to feed an infant who is working against a constant flow as delivered by the RAM cannulae is bound to cause problems. I don’t think it should be a surprise to find that trying to feed while struggling to breathe increases the risk of aspiration. Similarly, under treating a patient by placing them on nasal prongs would lead to increased work of breathing as while you may provide the needed O2 it is at lower lung volumes. Increasing work of breathing places infants at increased risk of aspiration. That is what I would take from this study. Interestingly, looking at the slide set from Calgary they did in fact use CPAP with the fluidic flip. Smart people they are. It would be too easy to embrace the results of this study and turn your nose to the SINC approach to feeding on CPAP. Perhaps somewhere out there someone will read this and think twice about abandoning the SINC approach and a baby will be better for it.
* SINC algorithm and picture of the fluidic flip courtesy of Stacey Dalgleish and the continued work of Alberta Health Services
by All Things Neonatal | Jan 11, 2017 | Infection
If you work in the NICU then you have seen your fair share of septic workups for late onset sepsis. Sepsis is such a common diagnosis that if I had to guess I would say that at least 50% of all discharge summaries would include this in a list of final diagnoses for any VLBW infant. If you were to look through the chart though you would find that while workups are common, the recovery of a pathogenic bacterium is not as much. This is in part due to the low threshold that many people have for doing such workups. A little bit of temperature instability, a few more apneic events than normal or a rise in O2 requirements may all trigger such investigations. When they come back negative we all feel good that we looked but we also are then quick to blame the etiology on something else. Mild fluctuations in temperature are written off as overbundling, apnea due to outgrowth of caffeine and a rise in FiO2 to evolving CLD. Maybe though the explanation at least in some cases is that there was a pathogen but we didn’t test for it.
Viruses are everywhere
Tis the season so to speak so everyone is on high alert for viruses in our homes, schools, malls etc but many of us consider the NICU to be mostly free of such pathogens. The truth is we mostly are provided that we all wash our hands well, keep sick contacts from visiting and put on a mask when our coughing starts. Alas, if you have done a handwashing audit as we have you would know that when looking at technique and duration of handwashing, we don’t always hit 100%. These audits are for health care practitioners but I have often wondered what sort of results we would see were we to do the same for parents and visitors. When we know the viruses are out there such as during outbreaks of RSV and influenza we can’t help but send off our samples for respiratory viruses more frequently but what if we did this with intention for every late onset septic workup?
Lucky For Us Someone Did Just That!
Back in 2014 the following study was published. Viral respiratory tract infections in the neonatal intensive care unit: the VIRIoN-I study. This was a simple prospective and elegant study in which any infant in the NICU who had never been home and was greater than 72 hours had respiratory samples sent for viral panels within 72 hours of starting antibiotics for presumed late onset sepsis. The findings were certainly interesting in that 6% of 135 sepsis evaluations tested positive for a virus. In the analysis, the infants had the following characteristics:
- tended to be older (41 vs 11 days; P = .007)
- exposed to individuals with respiratory tract viral symptoms (37% vs 2%; P = .003)
- lower total neutrophil counts (P = .02)
- best predictor of viral infection was the caregivers’ clinical suspicion of viral infection (P = .006)
What interests me about these results are a couple things. The first is that as I was once told, the sensitivity of asking if someone has been around sick people is low during peaks in viral outbreaks as who hasn’t? Perhaps what this study tells us is that within the NICU environment we actually do a reasonable job of keeping such contacts away but when they slip through infections happen. The second point worth mentioning is that a low neutrophil count is associated which is interesting given how often neutropenia is pointed to as a reason to start antibiotics. These viruses are troublesome creatures indeed!
Further Evidence Arrives
At the end of last year a similar study was published by the same group Viral Respiratory Infections in Preterm Infants during and after Hospitalization . They took a different approach this time out and took nasopharyngeal samples from 189 infants in the NICU (96 term and 93 preterm) within 7 days of birth and then sent samples weekly while in hospital followed by monthly for four months after discharge. In this collection of infants a mere 4 patients tested positive in NICU and all of them under 28 weeks of age at birth! How do we account for the remarkable reduction in risk while in hospital? To answer that you can read through the NICU environment in the full article if you have access. In short, they had a very rigorous infection control set of precautions set up. Interestingly only one of the infections was with RSV and the unit did not provide prophylaxis for infants in hospital. Perhaps with precautions like theirs they felt it was unnecessary. Once discharged a little over a third of patients acquired a viral infection in the first four months at home. Given the potential risk for readmission and with that to a PICU this rate of viral infection is concerning.
. They took a different approach this time out and took nasopharyngeal samples from 189 infants in the NICU (96 term and 93 preterm) within 7 days of birth and then sent samples weekly while in hospital followed by monthly for four months after discharge. In this collection of infants a mere 4 patients tested positive in NICU and all of them under 28 weeks of age at birth! How do we account for the remarkable reduction in risk while in hospital? To answer that you can read through the NICU environment in the full article if you have access. In short, they had a very rigorous infection control set of precautions set up. Interestingly only one of the infections was with RSV and the unit did not provide prophylaxis for infants in hospital. Perhaps with precautions like theirs they felt it was unnecessary. Once discharged a little over a third of patients acquired a viral infection in the first four months at home. Given the potential risk for readmission and with that to a PICU this rate of viral infection is concerning.
Vision for the future!
Taken together we can state that viruses do make their way into the NICU but fortunately not as commonly as one might think. What the last study in particular does remind us though is that we need to ensure that as part of discharge teaching parents take home many of the practices that we have used in the hospital with respect to hand hygiene, limiting visitors and not being afraid to holster some hand sanitizer for those times when soap and water are not so easy to come by. To be sure viruses are out there but at least for the first few months after discharge for our most vulnerable babies a little paranoia about viruses could go a long way.

by All Things Neonatal | Jan 5, 2017 | extubation, intubation
This is something that I continue to hear from time to time even in 2020 and I imagine I will continue to hear rumblings about this in 2022. Certainly, there are physical limitations when a baby is born at less than 500g. Have you tried fitting a mask to deliver NIPPV or CPAP to a baby this small? I have and it didn’t work. The mask was simply too big to provide a seal and while I am all for INSURE and emerging minimally invasive surfactant techniques they still require transitioning to a form of non invasive positive pressure ventilation to allow extubation success. Certainly though above the 500g barrier it may be that the greatest impediment to extubation is our own bias.
If this sounds a little familiar it is because I have written about this topic before Extubation failure is not a failure itself. The reason for bringing the topic up again though is that aside from needing to address our own fears there is a new systematic review that acts somewhat of a how to guide to optimizing your chance at a successful extubation. The review encompasses findings from 50 studies with successful extubation as defined as no need for reintubation within 7 days. Before getting into the details of the optimal approach it is worth reminding people that failure of extubation in even our smallest babies is not a failure itself. Such babies who “fail” up to 5 times do not suffer any long term consequences and may wind up with less risk of BPD than those who are kept intubated due to fear of failure.
So After Reviewing The Evidence What Are the Recipes To Success?
-
-
Continuous positive airway pressure
Reduced extubation failure in comparison with head-box oxygen (risk ratio [RR], 0.59;95%CI, 0.48-0.72; number needed to treat [NNT], 6; 95%CI, 3-9). If you aren’t extubating to nCPAP then chances are I would bet your success rates are quite low. Head boxes certainly can tell you how much O2 a patient requires but do nothing to help inflate alveolar spaces.
-
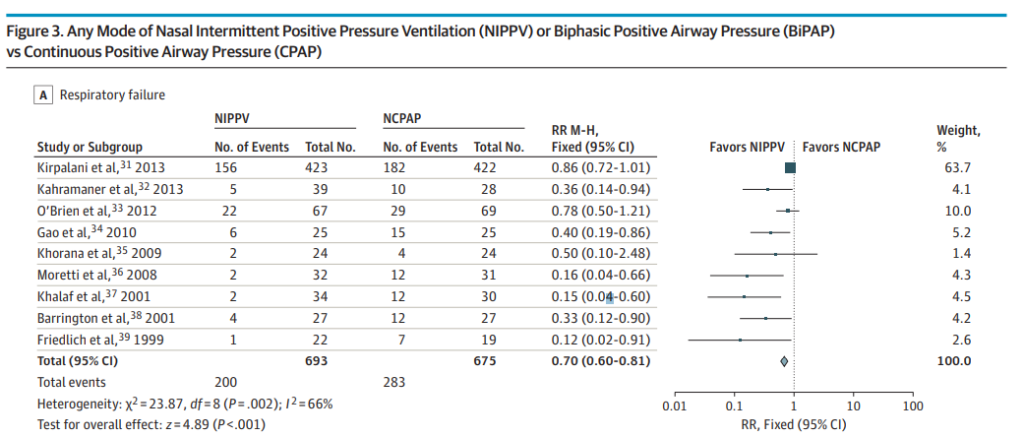
Nasal intermittent positive pressure ventilation (NIPPV) vs. CPAP
Higher prevention of extubation failure (RR, 0.70; 95%CI, 0.60-0.81; NNT, 8; 95%CI, 5-13). This one is of particular interest to me. The evidence has suggested this for some time and with a number needed to treat of 8 it would seem illogical to use anything else at the outset, especially in the smallest of infants. The issue here though is that at least here in Canada the options for delivering such NIPPV are currently quite limited. At the moment we are limited to use of ventilator NIPPV and the stability of the CPAP offered from such devices and the imposed work of breathing are most likely inferior to that found in variable flow devices which at this point have been pulled from the market. See Comparison of nasal continuous positive airway pressure delivered by seven ventilators using simulated neonatal breathing. What I hope 2017 brings is a comparison of the effectiveness of extubation success using new variable flow devices capable of generating previously unreachable CPAP pressures above 9 or 10 cm H2O. Will these attain similar effectiveness to the NIPPV devices? Forrest plot below
-
Methylxanthines reduced extubation failure (RR, 0.48; 95%CI, 0.32-0.71; NNT, 4; 95%CI, 2-7) compared with placebo or no treatment. Ok, pretty much anyone working in Neonatology would assume this but what really is at the crux of the discussion in 2016 and beyond is “what dose?” It has been pretty clear during my career thus far that there are some preterm infants that just don’t respond to conventional doses of caffeine base from 2.5 – 5 mg/kg/d. In our own units we have increased doses to 6, 7 or 8 mg/kg/d to achieve some degree of respiratory stimulation and usually been limited by tachycardia in determining how high we can go. Given the sparse literature regarding safety on this topic we are relegated to ask ourselves what is worse, leaving a baby on a ventilator or using higher doses of caffeine? I have given some thoughts on this before as well Are we overdosing preemies on caffeine?
-
Doxapram did not aid successful extubation (RR, 0.80; 95% CI, 0.22-2.97). For selfish reasons I have to admit I was happy to see this. We can’t access this medication very easily here in Canada so hearing that it doesn’t seem to work to enhance the likelihood of a successful extubation is somewhat of a relief.
A Cautionary Note
While I applaud the authors of the systematic review for performing such a thorough job I do feel the need to raise one concern with the analysis. It is not a major concern but one that I just feel the need to mention. Success if the studies was defined as not requiring reintubation within 7 days of extubation. My concern is that having such a lengthy time frame leaves the possibility that the decision to reintubate had nothing to do with the patient in fact not being ready. Seven days is a long time and much can happen in the life of a preterm infant in an NICU that triggers a reintubation. What if a patient needed to be transferred to a different NICU and for safe air transport it was deemed safest to replace the ETT? How many patients could have developed NEC or sepsis in the seven days? What if a PDA was being semi-electively ligated after a failed NSAID course?
In the end the impact of such conditions could be minimal but I am less convinced that a patient failed extubation when up to 7 days have passed. I would be very interested to see a similar study looking at a period of 24 or 48 hours after extubation and seeing how many stay that way. Would the predictors of success stay the same? Probably but I suspect the number safely extubated would rise as well.




 . They took a different approach this time out and took nasopharyngeal samples from 189 infants in the NICU (96 term and 93 preterm) within 7 days of birth and then sent samples weekly while in hospital followed by monthly for four months after discharge. In this collection of infants a mere 4 patients tested positive in NICU and all of them under 28 weeks of age at birth! How do we account for the remarkable reduction in risk while in hospital? To answer that you can read through the NICU environment in the full article if you have access. In short, they had a very rigorous infection control set of precautions set up. Interestingly only one of the infections was with RSV and the unit did not provide prophylaxis for infants in hospital. Perhaps with precautions like theirs they felt it was unnecessary. Once discharged a little over a third of patients acquired a viral infection in the first four months at home. Given the potential risk for readmission and with that to a PICU this rate of viral infection is concerning.
. They took a different approach this time out and took nasopharyngeal samples from 189 infants in the NICU (96 term and 93 preterm) within 7 days of birth and then sent samples weekly while in hospital followed by monthly for four months after discharge. In this collection of infants a mere 4 patients tested positive in NICU and all of them under 28 weeks of age at birth! How do we account for the remarkable reduction in risk while in hospital? To answer that you can read through the NICU environment in the full article if you have access. In short, they had a very rigorous infection control set of precautions set up. Interestingly only one of the infections was with RSV and the unit did not provide prophylaxis for infants in hospital. Perhaps with precautions like theirs they felt it was unnecessary. Once discharged a little over a third of patients acquired a viral infection in the first four months at home. Given the potential risk for readmission and with that to a PICU this rate of viral infection is concerning.
