by All Things Neonatal | May 30, 2018 | intubation, Neonatal, Neonatology, preemie, Prematurity, resuscitation
If I look back on my career there have been many things I have been passionate about but the one that sticks out as the most longstanding is premedicating newborns prior to non-emergent intubation. The bolded words in the last sentence are meant to reinforce that in the setting of a newborn who is deteriorating rapidly it would be inappropriate to wait for medications to be drawn up if the infant is already experiencing severe oxygen desaturation and/or bradycardia. The CPS Fetus and Newborn committee of which I am a member has a statement on the use of premedication which seems as relevant today as when it was first developed. In this statement the suggested cocktail of atropine, fentanyl and succinylcholine is recommended and having used it in our centre I can confirm that it is effective. In spite of this recommendation by our national organization there remain those who are skeptical of the need for this altogether and then there are others who continue to search for a better cocktail. Since I am at the annual conference for the CPS in Quebec city  I thought it would be appropriate to provide a few comments on this topic.
I thought it would be appropriate to provide a few comments on this topic.
Three concerns with rapid sequence induction (RSI) for premedication before intubation
1. “I don’t need it. I don’t have any trouble intubating a newborn” – This is perhaps the most common reason I hear naysayers raise. There is no question that an 60-90 kg practitioner can overpower a < 5kg infant and in particular an ELBW infant weighing < 1 kg. This misses the point though. Premedicating has been shown to increase success on the first attempt and shorten times to intubation. Dempsey 2006, Roberts 2006, Carbajal 2007, Lemyre 2009
2. “I usually get in on the first attempt and am very slick so risk of injury is less.” Not really true overall. No doubt there are those individuals who are highly successful but overall the risk of adverse events is reduced with premedication. (Marshall 1984, Lemyre 2009). I would also proudly add another Canadian study from Edmonton by Dr. Byrne and Dr. Barrington who performed 249 consecutive intubations with predication and noted minimal side effects but high success rates at first pass.
3. “Intubation is not a painful procedure”. This one is somewhat tough to obtain a true answer for as the neonate of course cannot speak to this. There is evidence available again from Canadian colleagues in 1984 and 1989 that would suggest that infants at the very least experience discomfort or show physiologic signs of stress when intubated using an “awake” approach. In 1984 Kelly and Finer in Edmonton published Nasotracheal intubation in the neonate: physiologic responses and effects of atropine and pancuronium. This randomized study of atropine with or without pancuronium vs control demonstrated intracranial hypertension only in those infants in the control arm with premedication ameliorating this finding. Similarly, in 1989 Barrington, Finer and the late Phil Etches also in Edmonton published Succinylcholine and atropine for premedication of the newborn infant before nasotracheal intubation: a randomized, controlled trial. This small study of 20 infants demonstrated the same finding of elimination of intracranial hypertension with premedication. At the very least I would suggest that having a laryngoscope blade put in your oral cavity while awake must be uncomfortable. If you still doubt that statement ask yourself whether you would want sedation if you needed to be intubated? Still feel the same way about babies not needing any?
4. What if I sedate and paralyze and there is a critical airway? Well this one may be something to consider. If one knows there is a large mass such as a cystic hygroma it may be best to leave the sedation or at least the paralysis out. The concern though that there might be an internal mass or obstruction that we just don’t know about seems a little unfounded as a justification for avoiding medications though.
Do we have the right cocktail?
The short answer is “I don’t know”. What I do know is that the use of atropine, an opioid and a muscle relaxant seems to provide good conditions for intubating newborns. We are in the era of refinement though and as a recent paper suggests, there could be alternatives to consider;Effect of Atropine With Propofol vs Atropine With Atracurium and Sufentanil on Oxygen Desaturation in Neonates Requiring Nonemergency IntubationA Randomized Clinical Trial. I personally like the idea of a two drug combination for intubating vs.. three as it leaves one less drug to worry about a medication error with. There are many papers out there looking at different drug combinations. This one though didn’t find a difference between the two combinations in terms of prolonged desaturations between the two groups which was the primary outcome. Interestingly though the process of intubating was longer with atropine and propofol. Given some peoples reluctance to use RSI at all, any drug combination which adds time to the the procedure is unlikely to go over well. Stay tuned though as I am sure there will be many other combinations over the next few years to try out!

by All Things Neonatal | Feb 16, 2018 | intubation, surfactant
A common concern in the NICU these days is the lack of opportunity to intubate. A combination of an increasing pool of learners combined with a move towards a greater reliance on non-invasive means of respiratory support is to blame in large part. With this trend comes a declining opportunity to practice this important skill and with it a challenge to get a tube into the trachea when it really counts. One such situation is a baby with escalating FiO2 requirements who one wishes to provide surfactant to. Work continues to be done in the area of aerosolized surfactant but as of yet this is not quite ready for prime time. What if there was another way to get surfactant to where it was needed without having to instill it directly into the trachea whether through a catheter (using minimally invasive techniques) or through an endotracheal tube?
Installation of surfactant into the trachea
Lamberska T et al have published an interesting pilot study looking at this exact strategy. Their paper entitled Oropharyngeal surfactant can improve initial stabilisation and reduce rescue intubation in infants born below 25 weeks of gestation takes a look at a strategy of instilling 1.5 mL of curosurf directly into the pharynx for infants 22-24 weeks through a catheter inserted 3-4 cm past the lips as a rapid bolus concurrent with a sustained inflation maneuver (SIM) of 25 cm of H2O for 15 seconds. Two more SIMs were allowed of the heart rate remained < 100 after 15 seconds of SIM. The theory here was that the SIM would trigger an aspiration reflex as the pressure in the pharynx increased leading to distribution of surfactant to the lung. The study compared three epochs from January 2011 – December 2012 when SIM was not generally practiced to July 2014 – December 2015 when SIM was obligatory. The actual study group was the period in between when prophylactic surfactant with SIM was practiced for 19 infants.
A strength of the study was that resuscitation practices were fairly standard outside of these changes in practice immediately after delivery and the decision to intubate if the FiO2 was persistently above 30% for infants on CPAP. A weakness is the size of the study with only 19 patients receiving this technique being compared to 20 patients before and 20 after that period. Not very big and secondly no blinding was used so when looking at respiratory outcomes one has to be careful to ensure that no bias may have crept in. If the researchers were strongly hoping for an effect might they ignore some of the “rules around intubation” and allow FiO2 to creep a little higher on CPAP as an example? Hard to say but a risk with this type of study.
What did they find?
The patients in the three epochs were no different from one and other with one potentially important exception. There were higher rates of antenatal steroid use in the study group (95% vs 75 and 80% in the pre and post study epochs). Given the effect of antenatal steroids on reducing respiratory morbidity, this cannot be ignored and written off.
Despite this difference it is hard to ignore the difference in endotracheal intubation in the delivery room with only 16% needing this in the study group vs 75 and 55% in the other two time periods. Interestingly, all of the babies intubated in the delivery area received surfactant at the same percentages as above. The need for surfactant in the NICU however was much higher in the study period with 79% receiving a dose in the study group vs 20 and 35% in the pre and post study groups. Other outcomes such as IVH, severe ROP and BPD were looked at with no differences but the sample again was small.
What can we take from this?
Even taking into account the effect of antenatal steroids, I would surmise that some surfactant did indeed get into the trachea of the infants in the study group. This likely explains the temporary benefit the babies had in the delivery suite. I suspect that there simply was not a big enough dose to fully treat their RDS leading to eventual failure on CPAP and a requirement for intubation. Is all lost though? Not really I think. Imagine you are in a centre where the Neonatologist is not in house and while he/she is called to the delivery they just don’t make it in time. The trainee tries to intubate but can’t get the tube in. Rather than trying several times and causing significant amounts of airway trauma (as well as trauma to their own self confidence) they could abandon further attempts and try instilling some surfactant into the pharynx and proving a SIM. If it works at all the baby might improve enough to buy some time for them to be stabilized on CPAP allowing time for another intubater to arrive.
While I don’t think there is enough here to recommend this as an everyday practice there just might be enough to use this when the going gets tough. No doubt a larger study will reveal whether there really is something here to incorporate into the tool chest that we use to save the lives of our smallest infants.
by All Things Neonatal | Apr 26, 2017 | intubation, resuscitation, Uncategorized
I think my first training in resuscitation began with the principles outlined in the NRP 3rd edition program. As we have moved through subsequent editions with the current edition being number 7, I can’t help but think about how many changes have occurred over that time. One such change has been the approach to using medications as part of a resuscitation. Gone are such things as calcium gluconate, naloxone and sodium bicarbonate but something that has stood the test of time is epinephrine. The dosing and recommendations for administering epinephrine have changed over time as well with the dose of endotracheal medication increasing from 0.01 to 0.03 and now to 0.05 – 0.1 mg/kg. While this dosing has increased, that of IV administration has remained the same at 0.01 to 0.03 mg/kg. The change in dosing for the ETT route was due to an increasing awareness that this route just isn’t as effective as IV. Having said that with only 0.1% of resuscitations requiring such support the experience with either route is fairly limited.
What is the concern?
Giving a medication directly via the IV route ensures the dose reaches the heart in the amount desired. In the case of ETT administration there are a few potential issues along the way. The first is that one needs to push the dose down the ETT and this presumes the ETT is actually in the trachea (could have become dislodged). Secondly, if the medication is sent to the lung what effect does the liquid component in the airways have in terms of dilution and distribution of the medication? Lastly, even if you get the epinephrine to the lung it must be picked up at the capillary level and then returned to the left side of the heart. In the absence of significant forward pulmonary blood flow this is not assured.
What is the evidence?
In terms of human clinical research it remains fairly limited. Barber published a retrospective review of 47 newborns who received epinephrine via the endotracheal route. The study Use and efficacy of endotracheal versus intravenous epinephrine during neonatal cardiopulmonary resuscitation in the delivery room found that spontaneous circulation was restored in 32% of this cohort. Following the first dose, a subsequent dose of intravenous epinephrine restored circulation in 77%. This study provided the first suggestion that the IV route may be better than endotracheal. Keep in mind though that this study was retrospective and as the authors conclude in the end, prospective studies are needed to confirm these findings. The question really is what is the likelihood of restoring circulation if the first dose is given IV?
Eleven years later we have a second study that attempts to answer this question although once again it is retrospective. Efficacy of Intravenous and Endotracheal Epinephrine during NeonatalCardiopulmonary Resuscitation in the Delivery Room by Halling et al. This study really was designed to answer two questions. The study group looked at the period from July 2006 to July 2014. During this period the dose of IV epinephrine remained unchanged as per NRP recommendations but the dose of endotracheal epinephrine increased from 0.01 to 0.03 and then to 0.05 mg/kg endotracheally. The increase was in response to both NRP and site observations that the lower doses were not achieving the effect they were hoping for.
The Results
|
ETT epinephrine |
IV Epinephrine |
| Number |
30 |
20 |
| Return of circulation |
23 |
15 |
| 1 dose |
6 |
4 |
| 2 dose |
5 |
8 |
| 3 doses |
9 |
0 |
| 4 doses |
3 |
3 |
In the ETT group all doses except for 3 after the first dose were given as IV. There was no difference in the response rate over time suggesting that higher doses do not truly increase the chance of a better response. The authors noted that the effectiveness of the two arms were not that different despite a significantly higher dose of epinephrine being administered to the group receiving ETT epinephrine first which is not surprising given the higher recommended dosages.
What I find interesting though is that giving the first dose of epinephrine was given IV in 20 of the paitents, if it is indeed the better route one might expect a better response than in the ETT group. The response from one dose of ETT epi was 20% while that from the IV first group was in fact also only 20%! We do indeed need to be careful here with small numbers but the results at least to me do not suggest strongly that giving IV epi first ensures success. What the study suggests to me is that two doses of epinephrine may be needed to restore circulation. If you choose to start with IV it certainly does not seem unwise but if you have any delays I don’t see any reason to avoid ETT epinephrine as your first line.
The reality is that for many individuals a UVC is a procedure that while they may have learned in an NRP class they may have never actually placed one. Having an ETT in place though seems like a good place to start. I doubt we will ever see a randomized trial of ETT vs IV epinephrine in Neonatology at this point given the stance by the NRP so these sorts of studies I suspect will be the best we get.
For now, based on what is out there I suggest use the route that you can get first but expect to need additional doses at least one more time to achieve success. Lastly remember that even if you do everything correct there will be some that cannot be brought back. Rest assured though that if the first dose was given via ETT you have still done your best if that was the route you had.

by All Things Neonatal | Jan 5, 2017 | extubation, intubation
This is something that I continue to hear from time to time even in 2020 and I imagine I will continue to hear rumblings about this in 2022. Certainly, there are physical limitations when a baby is born at less than 500g. Have you tried fitting a mask to deliver NIPPV or CPAP to a baby this small? I have and it didn’t work. The mask was simply too big to provide a seal and while I am all for INSURE and emerging minimally invasive surfactant techniques they still require transitioning to a form of non invasive positive pressure ventilation to allow extubation success. Certainly though above the 500g barrier it may be that the greatest impediment to extubation is our own bias.
If this sounds a little familiar it is because I have written about this topic before Extubation failure is not a failure itself. The reason for bringing the topic up again though is that aside from needing to address our own fears there is a new systematic review that acts somewhat of a how to guide to optimizing your chance at a successful extubation. The review encompasses findings from 50 studies with successful extubation as defined as no need for reintubation within 7 days. Before getting into the details of the optimal approach it is worth reminding people that failure of extubation in even our smallest babies is not a failure itself. Such babies who “fail” up to 5 times do not suffer any long term consequences and may wind up with less risk of BPD than those who are kept intubated due to fear of failure.
So After Reviewing The Evidence What Are the Recipes To Success?
-
-
Continuous positive airway pressure
Reduced extubation failure in comparison with head-box oxygen (risk ratio [RR], 0.59;95%CI, 0.48-0.72; number needed to treat [NNT], 6; 95%CI, 3-9). If you aren’t extubating to nCPAP then chances are I would bet your success rates are quite low. Head boxes certainly can tell you how much O2 a patient requires but do nothing to help inflate alveolar spaces.
-
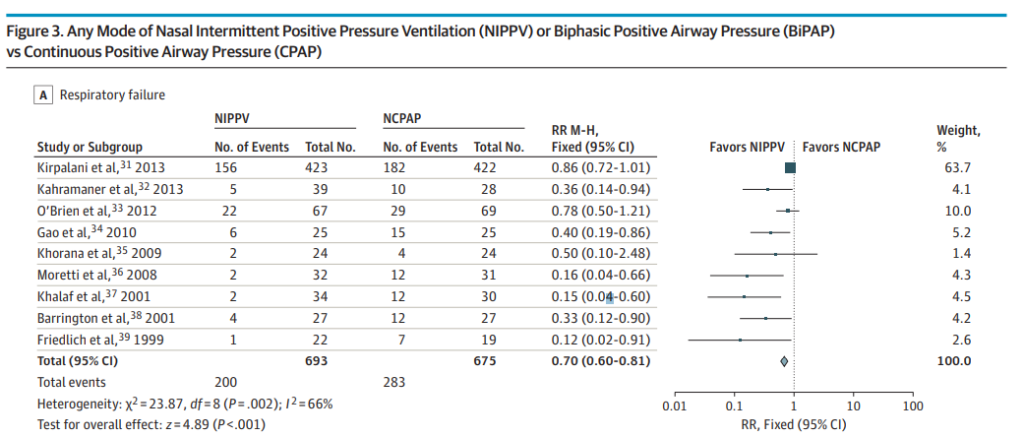
Nasal intermittent positive pressure ventilation (NIPPV) vs. CPAP
Higher prevention of extubation failure (RR, 0.70; 95%CI, 0.60-0.81; NNT, 8; 95%CI, 5-13). This one is of particular interest to me. The evidence has suggested this for some time and with a number needed to treat of 8 it would seem illogical to use anything else at the outset, especially in the smallest of infants. The issue here though is that at least here in Canada the options for delivering such NIPPV are currently quite limited. At the moment we are limited to use of ventilator NIPPV and the stability of the CPAP offered from such devices and the imposed work of breathing are most likely inferior to that found in variable flow devices which at this point have been pulled from the market. See Comparison of nasal continuous positive airway pressure delivered by seven ventilators using simulated neonatal breathing. What I hope 2017 brings is a comparison of the effectiveness of extubation success using new variable flow devices capable of generating previously unreachable CPAP pressures above 9 or 10 cm H2O. Will these attain similar effectiveness to the NIPPV devices? Forrest plot below
-
Methylxanthines reduced extubation failure (RR, 0.48; 95%CI, 0.32-0.71; NNT, 4; 95%CI, 2-7) compared with placebo or no treatment. Ok, pretty much anyone working in Neonatology would assume this but what really is at the crux of the discussion in 2016 and beyond is “what dose?” It has been pretty clear during my career thus far that there are some preterm infants that just don’t respond to conventional doses of caffeine base from 2.5 – 5 mg/kg/d. In our own units we have increased doses to 6, 7 or 8 mg/kg/d to achieve some degree of respiratory stimulation and usually been limited by tachycardia in determining how high we can go. Given the sparse literature regarding safety on this topic we are relegated to ask ourselves what is worse, leaving a baby on a ventilator or using higher doses of caffeine? I have given some thoughts on this before as well Are we overdosing preemies on caffeine?
-
Doxapram did not aid successful extubation (RR, 0.80; 95% CI, 0.22-2.97). For selfish reasons I have to admit I was happy to see this. We can’t access this medication very easily here in Canada so hearing that it doesn’t seem to work to enhance the likelihood of a successful extubation is somewhat of a relief.
A Cautionary Note
While I applaud the authors of the systematic review for performing such a thorough job I do feel the need to raise one concern with the analysis. It is not a major concern but one that I just feel the need to mention. Success if the studies was defined as not requiring reintubation within 7 days of extubation. My concern is that having such a lengthy time frame leaves the possibility that the decision to reintubate had nothing to do with the patient in fact not being ready. Seven days is a long time and much can happen in the life of a preterm infant in an NICU that triggers a reintubation. What if a patient needed to be transferred to a different NICU and for safe air transport it was deemed safest to replace the ETT? How many patients could have developed NEC or sepsis in the seven days? What if a PDA was being semi-electively ligated after a failed NSAID course?
In the end the impact of such conditions could be minimal but I am less convinced that a patient failed extubation when up to 7 days have passed. I would be very interested to see a similar study looking at a period of 24 or 48 hours after extubation and seeing how many stay that way. Would the predictors of success stay the same? Probably but I suspect the number safely extubated would rise as well.

 I thought it would be appropriate to provide a few comments on this topic.
I thought it would be appropriate to provide a few comments on this topic.



