by All Things Neonatal | Sep 13, 2017 | Neonatal, Neonatology, newborn, preemie, Prematurity, resuscitation, ventilation
We can always learn and we can always do better. At least that is something that I believe in. In our approach to resuscitating newborns one simple rule is clear. Fluid must be replaced by air after birth and the way to oxygenate and remove CO2 is to establish a functional residual capacity. The functional residual capacity is the volume of air left in the lung after a tidal volume of air is expelled in a spontaneously breathing infant and is shown in the figure. Traditionally, to establish this volume in a newborn who is apneic, you begin PPV or in the spontaneously breathing baby with respiratory distress provide CPAP to help inflate the lungs and establish FRC.
Is there another way?
Something that has been discussed now for some time and was commented on in the most recent version of NRP was the concept of using sustained inflation (SI) to achieve FRC. I have written about this topic previously and came to a conclusion that it wasn’t quite ready for prime time yet in the piece Is It Time To Use Sustained Lung Inflation In NRP?
The conclusion as well in the NRP textbook was the following:
“There are insufficient data regarding short and long-term safety and the most appropriate duration and pressure of inflation to support routine application of sustained inflation of greater than 5 seconds’ duration to the transitioning newborn (Class IIb, LOE B-R). Further studies using carefully designed protocols are needed”
So what now could be causing me to revisit this concept? I will be frank and admit that whenever I see research out of my old unit in Edmonton I feel compelled to read it and this time was no different. The Edmonton group continues to do wonderful work in the area of resuscitation and expand the body of literature in such areas as sustained inflation.
Can you predict how much of a sustained inflation is needed?
This is the crux of a recent study using end tidal CO2 measurement to determine whether the lung has indeed established an FRC or not. Dr. Schmolzer’s group in their paper (Using exhaled CO2 to guide initial respiratory support at birth: a randomised controlled trial) used end tidal CO2 levels above 20 mmHg to indicate that FRC had been established. If you have less CO2 being released the concept would be that the lung is actually not open. There are some important numbers in this study that need to be acknowledged. The first is the population that they looked at which were infants under 32 6/7 weeks and the second is the incidence of BPD (need for O2 or respiratory support at 36 weeks) which in their unit was 49%. This is a BIG number as in comparison for infants under 1500g our own local incidence is about 11%. If you were to add larger infants closer to 33 weeks our number would be lower due to dilution. With such a large number though in Edmonton it allowed them to shoot for a 40% reduction in BPD (50% down to 30%). To accomplish this they needed 93 infants in each group to show a difference this big.
So what did they do?
For this study they divided the groups in two when the infant wouldn’t breathe in the delivery room. The SI group received a PIP of 24 using a T-piece resuscitator for an initial 20 seconds. If the pCO2 as measured by the ETCO2 remained less than 20 they received an additional 10 seconds of SI. In the PPV group after 30 seconds of PPV the infants received an increase of PIP if pCO2 remained below 20 or a decrease in PIP if above 20. In both arms after this phase of the study NRP was then followed as per usual guidelines.
The results though just didn’t come through for the primary outcome although ventilation did show a difference.
| Outcome |
SI |
PPV |
p |
| BPD |
23% |
33% |
0.09 |
| Duration of mechanical ventilation (hrs) |
63 |
204 |
0.045 |
The reduction in hours of ventilation was impressive although no difference in BPD was seen. The problem though with all of this is what happened after recruitment into the study. Although they started with many more patients than they needed, by the end they had only 76 in the SI group and 86 in the PPV group. Why is this a problem? If you have less patients than you needed based on the power calculation then you actually didn’t have enough patients enrolled to show a difference. The additional compounding fact here is that of the Hawthorne Effect. Simply put, patients who are in a study tend to do better by being in a study. The observed rate of BPD was 33% during the study. If the observed rate is lower than expected when the power calculation was done it means that the number needed to show a difference was even larger than the amount they originally thought was needed. In the end they just didn’t have the numbers to show a difference so there isn’t much to conclude.
What I do like though
I have a feeling or a hunch that with a larger sample size there could be something here. Using end tidal pCO2 to determine if the lung is open is in and of itself I believe a strategy to consider whether giving PPV or one day SI. We already use colorimetric devices to determine ETT placement but using a quantitative measure to ascertain the extent of open lung seems promising to me. I for one look forward to the continued work of the Neonatal Resuscitation–Stabilization–Triage team (RST team) and congratulate them on the great work that they continue doing.
by All Things Neonatal | Sep 7, 2017 | preemie, Prematurity, ventilation
A grenade was thrown this week with the publication of the Australian experience comparing three epochs of 1991-92, 1997 and 2005 in terms of long term respiratory outcomes. The paper was published in the prestigious New England Journal of Medicine; Ventilation in Extremely Preterm Infants and Respiratory Function at 8 Years. This journal alone gives “street cred” to any publication and it didn’t take long for other news agencies to notice such as Med Page Today. The claim of the paper is that the modern cohort has fared worse in the long run. This has got to be alarming for anyone reading this! As the authors point out, over the years that are being compared rates of antenatal steroid use increased, surfactant was introduced and its use became more widespread and a trend to using non-invasive ventilation began. All of these things have been associated with better short term outcomes. Another trend was declining use of post-natal steroids after 2001 when alarms were raised about the potential harm of administering such treatments.
Where then does this leave us?
I suppose the first thing to do is to look at the study and see if they were on the mark. To evaluate lung function the study looked at markers of obstructive lung disease at 8 years of age in survivors from these time periods. All babies recruited were born between 22-27 completed weeks so were clearly at risk of long term injury. Measurements included FEV1, FVC, FVC:FEV1 and FEF 25-75%. Of the babies measured the only two significant findings were in the FEV1 and ratio of FEV1:FVC. The former showed a drop off comparing 1997 to 2005 while the latter was worse in 2005 than both epochs.
| Variable |
1991-92 |
1997 |
2005 |
| %predicted value |
N=183 |
N=112 |
N=123 |
| FEV1 |
87.9+/-13.4 |
92.0+/-15.7 |
85.4+/-14.4 |
| FEV1:FVC |
98.3+/-10 |
96.8+/-10.1 |
93.4+/-9.2 |
This should indeed cause alarm. Babies born in a later period when we thought that we were doing the right things fared worse. The authors wonder if perhaps a strategy of using more CPAP may be a possible issue. Could the avoidance of intubation and dependence on CPAP for longer periods actually contribute to injury in some way? An alternative explanation might be that the use of continuous oximetry is to blame. Might the use of nasal cannulae with temporary rises in O2 expose the infant to oxygen toxicity?
There may be a problem here though
Despite everyone’s best efforts survival and/or BPD as an outcome has not changed much over the years. That might be due to a shift from more children dying to more children living with BPD. Certainly in our own centre we have seen changes in BPD at 36 weeks over time and I suspect other centres have as well. With concerted efforts many centres report better survival of the smallest infants and with that they may survive with BPD. The other significant factor here is after the extreme fear of the early 2000s, use of postnatal steroids fell off substantially. This study was no different in that comparing the epochs, postnatal glucocorticoid use fell from 40 and 46% to 23%. One can’t ignore the possibility that the sickest of the infants in the 2005 cohort would have spent much more time on the ventilator that their earlier counterparts and this could have an impact on the long term lung function.
Another question that I don’t think was answered in the paper is the distribution of babies at each gestational age. Although all babies were born between 22-27 weeks gestational age, do we know if there was a skewing of babies who survived to more of the earlier gestations as more survived? We know that in the survivors the GA was not different so that is reassuring but did the sickest possible die more frequently leaving healthier kids in the early cohorts?
This bigger issue interestingly is not mentioned in the paper. Looking at the original cohorts there were 438 in the first two year cohort of which 203 died yielding a survival of 54% while in 1997 survival increased to 70% and in 2005 it was 65%. I can’t help but wonder if the drop in survival may have reflected a few more babies at less than 24 weeks being born and in addition the holding of post natal steroids leading to a few more deaths. Either way, there are enough questions about the cohorts not really being the same that I think we have to take the conclusions of this paper with a grain of salt.
It is a sensational suggestion and one that I think may garner some press indeed. I for one believe strongly though as I see our rates of BPD falling with the strategies we are using that when my patients return at 8 years for a visit they will be better off due to the strategies we are using in the current era. Having said that we do have so much more to learn and I look forward to better outcomes with time!

by All Things Neonatal | Aug 23, 2017 | donor milk, Neonatology, nutrition, preemie, Prematurity
Exclusive human milk (EHM) diets using either mother’s own milk or donor milk plus a human based human milk fortifier have been the subject of many papers over the last few years. Such papers have demonstrated reductions is such outcomes as NEC, length of stay, days of TPN and number of times feedings are held due to feeding intolerance to name just a few outcomes. There is little argument that a diet for a human child composed of human milk makes a great deal of sense. Although we have come to rely on bovine sources of both milk and fortifier when human milk is unavailable I am often reminded that bovine or cow’s milk is for baby cows.
Challenges with using an exclusive human milk diet.
While it makes intuitive sense to strive for an exclusive human milk diet, there are barriers to the same. Low rates of maternal breastfeeding coupled with limited or no exposure to donor breast milk programs are a clear impediment. Even if you have those first two issues minimized through excellent rates of breast milk provision, there remains the issue of whether one has access to a human based fortifier to achieve the “exclusive” human milk diet.
The “exclusive” approach is one that in the perfect world we would all strive for but in times of fiscal constraint there is no question that any and all programs will be questioned from a cost-benefit standpoint. The issue of cost has been addressed previously by Ganapathy et al in their paper Costs of Necrotizing Enterocolitis and Cost-Effectiveness of Exclusively Human Milk-Based Products in Feeding Extremely Premature Infants. The authors were able to demonstrate that choosing an exclusive human milk diet is cost effective in addition to the benefits observed clinically from such a diet. In Canada where direct costs are more difficult to visualize and a reduction in nursing staff per shift brings about the most direct savings, such an argument becomes more difficult to achieve.
Detractors from the EHM diet argue that we have been using bovine fortification from many years and the vast majority of infants regardless of gestational age have little challenge with it. Growth rates of 15-20 g/kg/d are achievable using such fortification so why would you need to treat all patients with an EHM diet?
A Rescue Approach
In our own centre we were faced with these exact questions and developed a rescue approach. The rescue was designed to identify those infants who seemed to have a clear intolerance to bovine fortifier as all of the patients we care for under 1250g receive either mother’s own or donor milk. The approach used was as follows:
A. < 27 weeks 0 days or < 1250 g
i. 2 episode of intolerance to HMF
ii. Continue for 2 weeks
This month we published our results from using this targeted rescue approach in Winnipeg, Human Based Human Milk Fortifier as Rescue Therapy in Very Low Birth Weight Infants Demonstrating Intolerance to Bovine Based Human Milk Fortifier with Dr. Sandhu being the primary author (who wrote this as a medical student with myself and others. We are thrilled to share our experience and describe the cases we have experienced in detail in the paper. Suffice to say though that we have identified value in such an approach and have now modified our current approach based on this experience to the following protocol for using human derived human milk fortifier in our centre to the current:
A. < 27 weeks 0 days or < 1250 g
i. 1 episode of intolerance to HMF
ii. Continue for 4 weeks
B. ≥ 27 week 0 days or ≥ 750g
i. 2 episodes of intolerance to HMF
ii. Continue for 4 weeks or to 32 weeks 0 days whichever comes sooner
We believe given our current contraints, this approach will reduce the risk of NEC, feeding intolerance and ultimately length of stay while being fiscally prudent in these challenging times. Given the interest at least in Canada with what we have been doing here in Winnipeg and with the publication of our results it seemed like the right time to share this with you. Whether this approach or one that is based on providing human based human milk fortifier to all infants <1250g is a matter of choice for each institution that chooses to use a product such as Prolacta. In no way is this meant to be a promotional piece but rather to provide an option for those centres that would like to use such products to offer an EHM diet but for a variety of reasons have opted not to provide it to all.
by All Things Neonatal | Aug 10, 2017 | newborn, preemie, Prematurity, resuscitation, ventilation
I know how to bag a baby. At least I think I do. Providing PPV with a bag-valve mask is something that you are taught in NRP and is likely one of the first skills you learned in the NICU. We are told to squeeze the bag at a rate of 40-60 breaths a minute. According to the Laerdal website, the volume of the preterm silicone bag that we typically use is 240 mL. Imagine then that you are wanting to ventilate a baby who is 1 kg. How much should you compress the bag if you wish to delivery 5 mL/kg. Five ml out of a 240 mL bag is not a lot of squeeze is it? Think about that the next time you find yourself squeezing one. You might then say but what about a t-piece resuscitator? A good choice option as well but how much volume are you delivering if you set the initial pressures at 20/5 for example? That would depend on the compliance of the lung of course. The greater the compliance the more volume would go in. Would it be 5 mL, 10 ml or even 2.5 mL based on the initial setting? Hard to say as it really depends on your seal and the compliance of the lung at the pressure you have chosen. If only we had a device that could deliver a preset volume just like on a ventilator with a volume guarantee setting!
Why is this holy grail so important?
It has been over 30 years since the importance of volutrauma was demonstrated in a rabbit model. Hernandez LA et al published Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. The study used three models to demonstrate the impact of volume as opposed to pressure on injuring the lung of preterm rabbits. Group 1 were rabbit ventilated at pressures of 15/30/45 cm H2O for one hour, group 2 rabbits with a cast around their thorax to limit volume expansion and group 3 sets of excised lungs with no restriction to distension based on the applied pressures. As you might expect, limitation of over distension by the plaster cast led the greatest reduction in injury (measured as microvascular permeability) with the excised lungs being the worst. In doing this study the authors demonstrated the importance of over distension and made the case for controlling volume more than pressure when delivering breaths to avoid excessive tidal volume and resultant lung injury.
The “Next Step” Volume Ventilator BVM
Perhaps I am becoming a fan of the Edmonton group. In 2015 they published A Novel Prototype Neonatal Resuscitator That Controls Tidal Volume and Ventilation Rate: A Comparative Study of Mask Ventilation in a Newborn Manikin. The device is tablet based and as described, rather than setting a PIP to deliver a Vt, a rate is set along with a volume to be delivered with a peep in this case set at +5. 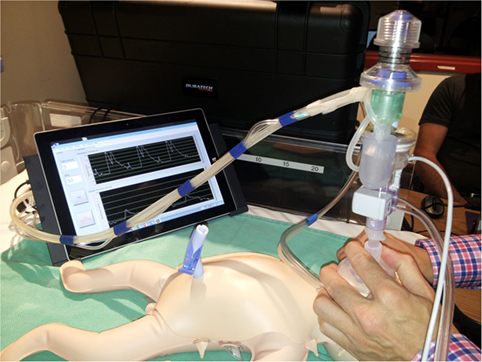 This study compared 5 different methods of delivering PPV to a 1 kg preterm manikin. The first was a standard self inflating bag, the next three different t-piece resuscitators and then the Next Step. For the first four the goal was to deliver a pressure of 20/5 at a rate of 40-60 breaths per minute. A test lung was connected to the manikin such that each device was used for a one minute period at three different levels of compliance (0.5 ml/cmH2O, 1.0 ml/cmH2O and then 2.0 ml/cm H2O representing increasing compliance. The goal of the study was to compare the methods in terms of delivering a volume of 5 mL to this 1 kg model lung. The order in which the devices were used was randomized for the 25 participants in the study who were all certified in NRP and included some Neonatologists.
This study compared 5 different methods of delivering PPV to a 1 kg preterm manikin. The first was a standard self inflating bag, the next three different t-piece resuscitators and then the Next Step. For the first four the goal was to deliver a pressure of 20/5 at a rate of 40-60 breaths per minute. A test lung was connected to the manikin such that each device was used for a one minute period at three different levels of compliance (0.5 ml/cmH2O, 1.0 ml/cmH2O and then 2.0 ml/cm H2O representing increasing compliance. The goal of the study was to compare the methods in terms of delivering a volume of 5 mL to this 1 kg model lung. The order in which the devices were used was randomized for the 25 participants in the study who were all certified in NRP and included some Neonatologists.
Some Concerning Findings
As I said at the beginning, we all like to think we know how to ventilate a newborn with BVM. The results though suggest that as compliance increases our ability to control how much volume we deliver to a lung based on a best guess for pressures needed is lacking. One caveat here is that the pressures set on the t-piece resucitators were unchanged during the 1 minute trials but then again how often during one minute would we change settings from a starting point of 20/5?
|
|
Vt (mL) |
|
|
0.5 mL/cmH20 |
1.0 mL/cmH20 |
2.0mL/cmH20 |
| Self inflating |
11.4 |
17.6 |
23.5 |
| Neo-Tee |
5.6 |
11.2 |
19.3 |
| Neopuff |
6.1 |
10 |
21.3 |
| Giraffe |
5.7 |
10.9 |
19.8 |
| Next Step |
3.7 |
4.9 |
4.5 |
Without putting in all the confidence intervals I can tell you that the Next Step was the tightest. What you notice immediately (or at least I did) was that no matter what the compliance, the self inflating bag delivers quite an excessive volume even in experienced hands regardless of compliance. At low compliance the t-piece resuscitators do an admirable job as 5-6 ml/kg of delivered Vt is reasonable but as compliance improves the volumes increase substantially. It is worth pointing out that at low compliance the Next Step was unable to deliver the prescribed Vt but knowing that if you had a baby who wasn’t responding to ventilation I would imagine you would then try a setting of 6 ml/kg to compensate much like you would increase the pressure on a typical device. How might these devices do in a 29 week infant for example with better compliance than say a 24 week infant? You can’t help but wonder how many babies are given minutes of excessive Vt after birth during PPV with the traditional pressure limited BVM setup and then down the road how many have BPD in part because of that exposure.
I wanted to share this piece as I think volume resuscitation will be the future. This is just a prototype or at least back then it was. Interestingly in terms of satisfaction of use, the Next Step was rated by the participants in the study as being the easiest and most comfortable to use of all the devices studied. Adding this finding to the accuracy of the delivered volume and I think we could have a winner.
by All Things Neonatal | Aug 2, 2017 | Neonatal, Neonatology, newborn, Pain in the Neonate, preemie
I would consider myself fairly open minded when it comes to care in the NICU. I wouldn’t call myself a maverick or careless but I certainly am open to new techniques or technologies that may offer a better level of care for the babies in our unit. When it comes to “non-Western” concepts though such as therapeutic touch, chiropractic manipulations of infants and acupuncture (needle or otherwise) I have generally been a skeptic. I have written about such topics before with the most popular post being Laser acupuncture for neonatal abstinence syndrome. My conclusion there was that I was not a fan of the strategy but perhaps I could be more open to non traditional therapies.
Magnetic Acupuncture
This would appear to be the newest and perhaps strangest (to me at least) approach to pain relief that I have seen. I do love name of this study; the MAGNIFIC trial consisted of a pilot study on the use of auricular magnetic acupuncture to alleviate pain in the NICU from heel lances. The study was published in Acta Paediatrica this month; Magnetic Non-Invasive Acupuncture for Infant Comfort (MAGNIFIC) – A single-blinded randomized controlled pilot trial. The goal here was to measure pain scores using the PIPP scoring system for pain in the neonate before during and after a painful experience (heel lance) in the NICU. Being a pilot study it was small with only 20 needed per arm based on the power calculation to detect a 20% difference in scores. The intervention used small magnets placed at specific locations on the ear of the infant at least two hours before the heel lance was to occur. Before I get into the results, the authors of the study provide references to explain how the therapy works. Looking at the references I have to admit I was not able to obtain complete papers but the evidence is generally it would appear from adult patients. The explanation has to do with the magnetic field increasing blood flow to the area the magnet is applied to and in addition another reference suggests that there are affects the orbitofrontal and limbic regions which then impacts neurohormonal responses as seen in functional MRI. The evidence to support this is I would have thought would be pretty sparse but I was surprised to find a literature review on the subject that looked at 42 studies on the topic. The finding was that 88% of the studies reported a therapeutic effect. The conclusion though of the review was that the quality of the included studies was a bit sketchy for the most part so was not able to find that this should be a recommended therapy.
So what were the results?
Despite my clear skepticism what this study did well was that aside from the magnets, the intervention was the same. Twenty one babies received the magnetic treatments vs 19 placebo. There was a difference in the gestational ages of the babies with the magnet treated infants being about two weeks older (35 vs 33 weeks). What difference that might in and of itself have on the PIPPs scoring I am not sure. The stickers were applied to the ears with and without magnets in a randomized fashion and the nurses instructed to score them using the PIPP scoring system. Interestingly, as per their unit policy all babies received sucrose as well before the intervention of a heel lance so I suppose the information gleaned here would be the use of magnets as an adjunctive treatment. No difference was noted in the two groups before and after the heel lance but during the procedure the magnet treated infants had a difference in means (SD): 5.9 (3.7) v 8.3 (4.7), p=0.04). No differences were found in secondary measures such as HR or saturation and no adverse effects were noted. The authors conclusions were that it was feasible and appears safe and as with most pilot studies warrants further larger studies to verify the results.
Should we run out and buy it?
One of the issues I have with the study is that in the introduction they mention that this treatment might be useful where kangaroo care (KC) is not such as a critically ill infant. Having placed infants who are quite sick in KC and watched wonderful stability arise I am not sure if the unit in question under utilizes this important modality for comfort.
The second and perhaps biggest issue I have here is that although the primary outcome was reached it does seem that there was some fishing going on here. By that I mean there were three PIPP scores examined (before, during and after) and one barely reached statistical significance. My hunch is that indeed this was reached by chance rather than it being a real difference.
The last concern is that while the intervention was done in a blinded and randomized fashion, the evidence supporting the use of this in the first place is not strong. Taking this into account and adding the previous concern in as well and I have strong doubts that this is indeed “for real”. I doubt this will be the last we will hear about it and while my skepticism continues I have to admit if a larger study is produced I will be willing and interested to read it.

by All Things Neonatal | Mar 22, 2017 | preemie, transfusion
A debate broke out recently at one of our rounds when someone asked whether a recent case of NEC was possibly related to a transfusion that a baby received. Much has been written about Transfusion Associated Necrotizing Enterocolitis (TANEC) with the pendulum swinging back and forth between it existing as a real entity or simply being an association that is not causative in the least. Using one of my favourite sources, a retrospective analysis of the Canadian Neonatal Network database found no difference in mortality or morbidities for those who had a transfusion and NEC vs those without. Despite this we continue to see those who “hold feeds” for a few hours prior to transfusion and then resume them a few hours later. Why does this happen?
Risk vs Benefit
Those who hold feeds argue that in Neonatology we hold feeds for far less. Furthermore, what is the harm? If a baby develops NEC within 24 hours of a transfusion and we held the feeds we feel we have done all we could. If a baby is fed and develops NEC we are left asking “what if?”. The purists out there would argue the contrary though, that the evidence is not strong enough to support the practice and may require the insertion of an IV which is a painful procedure and places the infant at risk of infection from one or more skin breaks. Additionally, does the interruption of feeds potentially alter the microbiome of the patient and with it risk potential downstream consequences. In case you are wondering, I have tended to sit on the side of holding a feed although more often when I am asked about it than ordering it upfront. The fact is I just don’t know. The evidence has never been solid in this regard but it is hard to ignore the possibility when you have been bitten once or twice before (whether it was causative or not!). I doubt it really exists but then again what if there is something there?
It May Not Be The Transfusion But Anemia Itself
A recent paper Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants may have found a possible explanation to the ongoing debate. Research papers associating transfusions with NEC may all have one thing in common in that they have not been able to prove causation. When you have many papers finding the same thing it leads medical teams to begin to believe there is causation. Something else may be at play at this paper suggests another association which again may not be causative but at least in my mind is perhaps biologically plausible. It may be that those patients who are transfused when their hemoglobin is below a threshold of 80 g/L are at increased risk of developing NEC rather than all patients transfused.
This study was a secondary analysis of a prospective study on transfusion transmission of cytomegalovirus in preterm infants < 1500g. The authors chose 80 g/L as a cutoff based on previous studies suggesting this threshold as an important one for transfusion practices. Forty eight out of 60 eligible infants developed NEC and it is from this 48 that multivariable analysis sought to identify factors predisposing to the outcome in question of NEC. The factor with the greatest hazard risk for NEC was severe anemia in a given week with an approximate 6 fold risk (range 2 – 18) while receiving an RBC transfusion in a given week of life did not meet statistical significance.
What does this mean?
Before embracing the result and concluding we have the answer we have to acknowledge the authors have gone on a fishing expedition of sorts. Any secondary analysis of a study that is done carries with it some words of warning. There may be variables that were not controlled for that are affecting the results. As well when looking at many many variables it could be by chance that something or several things come up by chance. Lastly it may be that again there is nothing more than an association here at play. Having said that, there is some biologic plausibility at least here.
- Delivery of oxygen to the tissues is dependent on HgB level. The oxygen content of blood is described by: O2 content = (gm Hbg)(1.34 ml O2/gm Hbg)(% sat) + 0.003(pO2) = ml O2/dL.
- Oxygen delivery = cardiac output X O2 concentration (or content)
- Could RBCs become less deformable and increase viscosity in low O2 environments? This could be the case when the HgB declines below 80 g/L. Such changes to deformability have been demonstrated at mild levels of hypoxia as might exist in low pO2 conditions at the tissue level with anemia.
So imagine we have fewer RBCs carrying as much oxygen as they can but eventually you cross a threshold where there is not enough O2 being delivered at the tissue level and the RBCs become lodged or perhaps sluggish as they move through capillaries of the intestines. Add to this that NEC occurs in watershed areas most commonly and you have the potential setup for NEC.
Can we use the results of this study?
I suppose statistical purists out there will argue that it is merely an association. The fact remains that there are many people who are holding feeds for varying amounts of time despite the lack of conclusive evidence that TANEC exists. I wonder if a middle ground might be to be more cautious and restrict such practice to those with low HgB values below 80 g/L as the authors here have found. To me at least there is biologic plausibility as outlined above. It would seem to me that to hold feeds for all babies is excessive and likely without evidence but could the threshold actually matter which it comes to oxygen content. Given that NEC is a condition related to ischemia, the authors here have provided another association that makes me at the very least scratch my head.





 This study compared 5 different methods of delivering PPV to a 1 kg preterm manikin. The first was a standard self inflating bag, the next three different t-piece resuscitators and then the Next Step. For the first four the goal was to deliver a pressure of 20/5 at a rate of 40-60 breaths per minute. A test lung was connected to the manikin such that each device was used for a one minute period at three different levels of compliance (0.5 ml/cmH2O, 1.0 ml/cmH2O and then 2.0 ml/cm H2O representing increasing compliance. The goal of the study was to compare the methods in terms of delivering a volume of 5 mL to this 1 kg model lung. The order in which the devices were used was randomized for the 25 participants in the study who were all certified in NRP and included some Neonatologists.
This study compared 5 different methods of delivering PPV to a 1 kg preterm manikin. The first was a standard self inflating bag, the next three different t-piece resuscitators and then the Next Step. For the first four the goal was to deliver a pressure of 20/5 at a rate of 40-60 breaths per minute. A test lung was connected to the manikin such that each device was used for a one minute period at three different levels of compliance (0.5 ml/cmH2O, 1.0 ml/cmH2O and then 2.0 ml/cm H2O representing increasing compliance. The goal of the study was to compare the methods in terms of delivering a volume of 5 mL to this 1 kg model lung. The order in which the devices were used was randomized for the 25 participants in the study who were all certified in NRP and included some Neonatologists.