
by All Things Neonatal | Jul 18, 2019 | ventilation
To be sure there are fans of both HFNC and CPAP out there. I have often heard from other Neonatologists that they use HFNC and find positive results while other centres refuse to use it in favour of the tried and true CPAP. Turning to the literature you will find some conflicting results with some studies suggesting equity and others more recently favouring CPAP. There has been speculation as to why one would be superior to the other and now we appear to have some answers as to where the differences lie.
A Physiologic Study
Liew et al published Physiological effects of high-flow nasal cannula therapy in preterm infants this month in an elegant study of 40 infants. The study was fairly simple in design either randomizing infants <37 weeks to starting with nCPAP +6 and then transitioning to 8 l/min HFNC followed by stepwise reductions of 1 l/min until 2 l/min was reached or the reverse, starting with 2 l/min and working their way up and then transitioning to nCPAP+6. All infants were on one or the other modality at the start and were all at least 3 days old, they were randomized to one or the other arm regardless of where they started off. Physiologic measurements were taken at each step including the following:
Mv -Minute ventilation
pEEP – nasopharyngeal end-expiratory pressure
pEECO2 -nasopharyngeal end-expiratory CO2
RR – respiratory rate;
SpO2 – oxygen saturation
TCCO2 – transcutaneous CO2
Vt – tidal volume
A Fabian device was used to deliver either HFNC or CPAP at the different flows for all patients.
The Results
The authors certainly found some interesting results that I think shed some light on why comparisons of HFNC and CPAP have been so inconsistent.
Table 2 contains the results of the study and I will point out the main findings below.
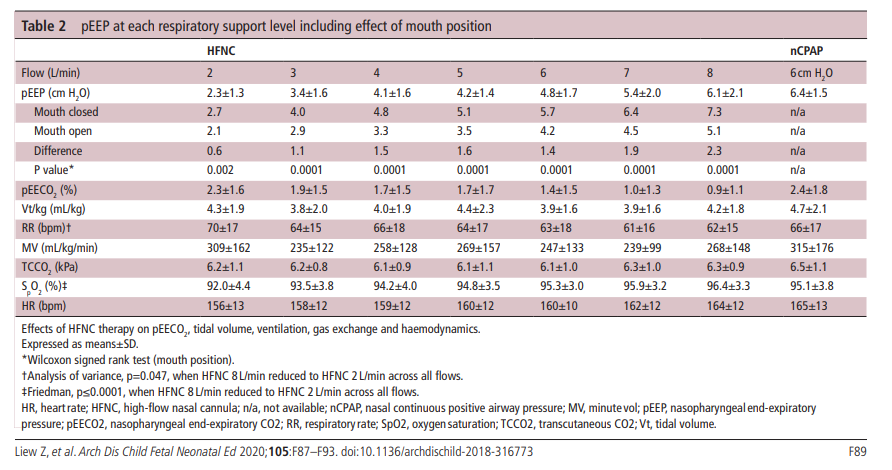

1. Flow matters – Compared to nCPAP+6 which is fairly consistent flows below 6 l/min deliver pEEP that is below 6 cm H2O.
2. Keep the mouth shut – With CPAP whether the mouth is open or closed the Fabian device delivers +6 cm H2O. As you can see from the table, when the mouth is open transmitted pressures drop off substantially. The infant put on a flow of even 6-8 l/min of HFNC sees pressures less than +6 consistently.
3. As flows increase end expiratory CO2 decreases. HFNC seems to help wash out CO2
4. Low flow rates on HFNC do not seem to help with ventilation as much as higher flow rates. In order to maintain Mv these infants at 2 l/min flow become tachypneic. The low pressures produced likely cause some atelectasis and hence tachypnea.
Size matters! Beware of excessive pressures.
An additional finding of this study was that on “multiple linear regression, flow rate, mouth position, current weight and gestation but not prong-to-nares ratio significantly predicted pEEP and account for a significant amount of its variance (F(4431)=143.768, p<0.0001), R2=0.572, R2=adjusted 0.568).” Essentially, infants under 1000g in particular could see pEEP levels as high as 13 cm H2O with flows of 8 l/min. The variability in transmitted pressures with HFNC is shown nicely in this figure from the study.
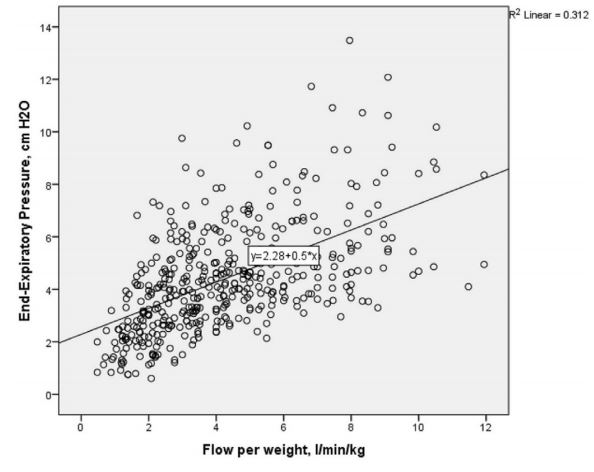

As flows increase above 6 l/min the actual pressures delivered become less reliable.
Conclusions
Looking at this data, it becomes evident why HFNC may be failing in its attempt to dethrone nCPAP. In order to achieve higher pressures and provide comparable distending pressure to nCPAP you need higher flows. With higher flows though come the problem of greater variability in delivered pressure. While the average pressure delivered may be equivalent or even higher than a CPAP of +6, in some infants (especially those below 1000g) one may be delivering significantly higher pressures than intended which may help with oxygenation and preventing intubation but others may be seeing far less than needed.
What it comes down to is that nCPAP is better at delivering a consistent amount of pressure. Studies using lower flows of HFNC likely failed to show superiority to CPAP as they just didn’t deliver enough pressure. An example of this was the study by Roberts CT et al Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants, in which flows of 6-8 l/min were used. Other studies using higher pressures could have been problematic due to open mouths, or larger babies not receiving as much benefit.
I am not saying that we should throw out HFNC entirely however. Depending on the unit you practice in you might not be able to use CPAP but HFNC may be allowed. If you had to choose between no support or HFNC I would likely go with the HFNC. For me at least, if I want to delivery reliable pressures in my tertiary care NICU I will be calling for the CPAP.
by All Things Neonatal | Sep 29, 2016 | ventilation
This may sound familiar as I wrote about this topic in the last year but the previous post was restricted to infants who were under 1000g. High Flow Nasal Cannula be careful out there had a main message that suggested the combined outcome of BPD or death was more prevalent when HFNC is used alone or with CPAP than when CPAP is used alone. The question remains though whether this applies to larger infants. Without looking at the evidence for that combined outcome most people would say there is unlikely to be a difference. Larger more mature babies have a much lower risk of BPD or death so proponents of HFNC would say it is simpler to use and helps prevent nasal breakdown as well. The question remains as to whether all outcomes are the same in larger infants and that is the point of this post.
A Non-Inferiority Trial
First off it is important to understand what this type of trial is. The first requirement is that the two treatments have both been compared to a placebo and found to be both effective. Once you establish that you have a choice between two treatment options then you eliminate the placebo and compare them head to head. What you are looking for in this type of trial is to determine not whether one is better than the other but that there is no difference in a clinical outcome of interest. If you find no difference then the next step is to look at other outcomes that might be of interest and see if there are any benefits to picking one versus the other. In the case of CPAP vs HNFC, if a non-inferiority trial showed no difference in an important outcome such as length of stay but nasal breakdown was less with HFNC it might lead a unit to use HFNC for their infants. Okay, now that we have that cleared up we can move on to an actual study examining this very subject.
This was an interesting study with a great name (The HIPSTER trial) that enrolled infants > 28 weeks and 0 days with none of the infants receiving surfactant but either being randomized to HFNC or CPAP after delivery. These infants were your typical modern day cohort of babies who may avoid intubation and surfactant by establishing FRC early with positive pressure applied to the nose through one of these devices. The end point for the study was treatment failure within 72 hours. If an infant failed in the HFNC they could have a trial of CPAP whereas in the CPAP group they were intubated. For each infant in the HFNC group flow was set from 6-8 l/min and for CPAP 6-8 cmH2O.
Treatment was considered to have failed if an infant receiving maximal support (high-flow therapy at a gas flow of 8 liters per minute or CPAP at a pressure of 8 cm of water) met one or more of the following criteria:
- FiO2 of 0.4 or higher
- Arterial or free flowing cap gas with a pH of 7.2 or less plus a pCO2 > 60 mm Hg obtained at least 1 hour after starting treatment
- Two or more episodes of apnea requiring positive-pressure ventilation within a 24-hour period or six or more episodes requiring any intervention within a 6-hour period.
- Infants with an urgent need for intubation and mechanical ventilation.
So what happened?
The trial randomized 583 infants (278 HFNC, 286 CPAP) but was halted by the data and safety monitoring committee after an analysis of the first 515 revealed that the outcome was worse in the HFNC group (25.5% failure rate vs 13.3 for CPAP). Interestingly treatment failures were more common in babies below and above 32 weeks so it was not just the smallest infants who failed.
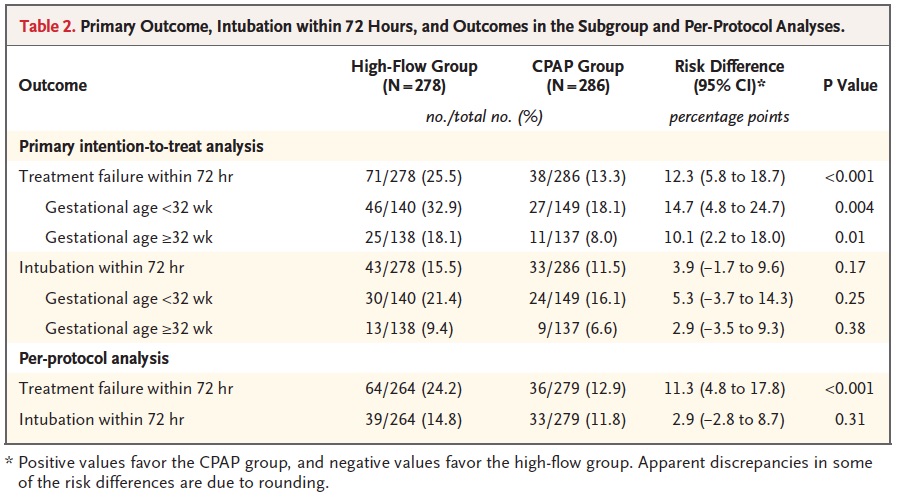 Another interesting finding was that the most common reason for treatment failure was criteria 1 (FiO2 > 40%) while intubation was higher for all infants but did not reach statistical significance. Curiously what did reach a significant difference was criteria #4 (18.4% urgent intubations in the CPAP group vs 5.6% in the HFNC group). You might be tempted to therefore ponder which is worse, a little O2 or being intubated but you need to recall the trial design which was set up to provide this kind of result. If you failed HFNC you were placed on CPAP whereas if you failed CPAP you were intubated. In the HFNC group, 78 infants were deemed to have failed but 28 of them were in fact “rescued by CPAP”. It therefore isn’t a fair comparison when it comes to urgent intubation since if you failed CPAP there wasn’t another option.
Another interesting finding was that the most common reason for treatment failure was criteria 1 (FiO2 > 40%) while intubation was higher for all infants but did not reach statistical significance. Curiously what did reach a significant difference was criteria #4 (18.4% urgent intubations in the CPAP group vs 5.6% in the HFNC group). You might be tempted to therefore ponder which is worse, a little O2 or being intubated but you need to recall the trial design which was set up to provide this kind of result. If you failed HFNC you were placed on CPAP whereas if you failed CPAP you were intubated. In the HFNC group, 78 infants were deemed to have failed but 28 of them were in fact “rescued by CPAP”. It therefore isn’t a fair comparison when it comes to urgent intubation since if you failed CPAP there wasn’t another option.
Not a total loss
Nasal trauma was indeed much lower in the HFNC group, occurring only 8.3% vs 18.5% of the time with CPAP. Pneumothorax was also found to be significantly different with none of the patients in the HFNC group having that complication vs 2.1% in the CPAP group. What this study tells us is that as a primary modality to treat newborns with RDS who have not received surfactant it is preferable to use CPAP in the first 72 hours. Some of you may say it might not say that at all but consider the impact of having more babies exposed to high FiO2. We know from other studies that high FiO2 can be quite damaging to preterm infants and this study was certainly not powered to look at all those important outcomes such as ROP, PVL and BPD. The authors report them and found no difference but without adequate power to show a difference I wouldn’t take much comfort in those findings.
I think were things may settle out though is what to do in more mature infants. There is no question that for those on chronic respiratory support there is some risk of nasal breakdown. Although I don’t have much experience with HFNC I would think that for the older patient who either already has BPD at 36 weeks or is close to that point but reliant on +4 or +5 CPAP that HFNC might help “give them a break”. As such I don’t see this as a total loss but rather an option to try when CPAP for whatever reason is not tolerated.
As a primary therapy for non-invasive management RDS I will keep my CPAP for all babies thank you.
by All Things Neonatal | Jun 15, 2016 | Uncategorized, ventilation
As the saying goes the devil is in the details. For some years now many centres worldwide have been publishing trials pertaining to high flow nasal cannulae (HFNC) particularly as a weaning strategy for extubation. The appeal is no doubt partly in the simplicity of the system and the perception that it is less invasive than CPAP. Add to this that many centres have found less nasal breakdown with the implementation of HFNC as standard care and you can see where the popularity for this device has come from.
This year a contact of mine Dominic Wilkinson on twitter (if you don’t follow him I would advise having a look!) published the following cochrane review, High flow nasal cannula for respiratory support in preterm infants. The review as with most cochrane systematic reviews is complete and comes to a variety of important conclusions based on 6 studies including 934 infants comparing use of HFNC to CPAP.
1. No differences in the primary outcomes of death (typical RR 0.77, 95% CI 0.43 to 1.36; 5 studies, 896 infants) or CLD.
2. After extubation to HFNC no difference in the rate of treatment failure (typical RR 1.21, 95% CI 0.95 to 1.55; 5 studies, 786 infants) or reintubation (typical RR 0.91, 95% CI 0.68 to 1.20; 6 studies, 934 infants).
3. Infants randomised to HFNC had reduced nasal trauma (typical RR 0.64, 95% CI 0.51 to 0.79; typical risk difference (RD) -0.14, 95% CI -0.20 to -0.08; 4 studies, 645 infants).
4. Small reduction in the rate of pneumothorax (typical RR 0.35, 95% CI 0.11 to 1.06; typical RD -0.02, 95% CI -0.03 to -0.00; 5 studies 896 infants) in infants treated with HFNC but the RR crosses one so this may be a trend at best.
If one was to do a quick search for the evidence and found this review with these findings it would be very tempting to jump on the bandwagon. Looking at the review a little closer though there is one line that I hope many do not miss and I was happy to see Dominic include it.
“Subgroup analysis found no difference in the rate of the primary outcomes between HFNC and CPAP in preterm infants in different gestational age subgroups, though there were only small numbers of extremely preterm and late preterm infants.”
In his conclusion he further states:
Further evidence is also required for evaluating the safety and efficacy of HFNC in extremely preterm and mildly preterm subgroups, and for comparing different HFNC devices.
With so few ELBW infants included and with these infants being at highest risk of mortality and BPD our centre has been reluctant to adopt this mode of respiratory support in the absence of solid evidence that it is equally effective to CPAP in these smallest infants. A big thank you to our Respiratory Therapy Clinical Specialist for harping on this point over the years as the temptation to adopt has been strong as other centres turn to this strategy.
Might Not Be So Safe After All
Now do not take what I am about to say as a slight against my twitter friend. The evidence to date points to exactly what he and his other coauthors concluded but with the release of an important paper in May by Taka DK et al, I believe caution is needed when it comes to our ELBW infants.
High Flow Nasal Cannula Use Is Associated with Increased Morbidity and Length of Hospitalization in Extremely Low Birth Weight Infants
This paper adds to the body of literature on the topic as it truly focuses on the outcome of infants < 1000g. While this study is retrospective in nature it does cover a five year period and examines important outcomes of interest to this population.
The primary outcome in this case was death or BPD and whether HFNC was used alone or with CPAP, this was more frequent than when CPAP was used alone. Other important findings were the need for multiple and longer courses of ventilation in those who received at least some HFNC. In these times of overburdened health care systems with goals of improving patient flow, it is also worth noting that there was a significant prolongation of length of stay with use of HFNC or HFNC and CPAP.
One interesting observation was that the group that fared the worst across the board was the combination of CPAP and HFNC rather than HFNC alone.
|
CPAP (941) |
HFNC (333) |
HFNC +/- CPAP (1546) |
| CPAP d (median, IQR) |
15(5-28) |
|
7 (1-19) |
| HFNC d (median, IQR) |
14(5-25) |
13 (6-23) |
| HFNC +/- CPAP |
15 (5-28) |
14(5-25) |
26 (14-39) |
| BPD or death % |
50.40% |
56.80% |
61.50% |
| BPD % |
42.20% |
52.20% |
59.00% |
| Multiple ventiation courses |
51.10% |
53.10% |
64.70% |
| More than 3 vent courses |
17.60% |
21.00% |
29.40% |
| Ventilator d (median, IQR) |
18(5-42) |
25 (6-52) |
30 (10-58) |
I believe the finding may be explained by the problem inherent with retrospective studies. This is not a study in which patients were randomized to either CPAP, HFNC or CPAP w/HFNC. If that were the case one would expect lung pathologies and severity of illness to even ou,t such that differences between groups might be explained by the difference in treatments. In this study though we are looking though the rearview mirror so to speak. How could we account for the combination being worse than the HFNC alone? I suspect it relates to the severity of lung disease. The babies who were placed on HFNC and did well on it might have had less severe chronic changes. What might be said about those that had the combination? Well, one could postulate that there might be some who were extubated to HFNC and collapsed needing escalation to CPAP and then failing that therapy were reintubated. Another explanation could be those babies who were placed on CPAP after extubation and transitioned before their lungs were ready to HFNC may have failed and lost FRC thereby going back to CPAP and possibly intubation. Exposure in either circumstance to HFNC would therefore put them at risk of further positive pressure ventilation and subsequent further lung injury. The babies who could tolerate transition to HFNC without CPAP might be intermediary in their outcomes (as they were found to be) as they lost FRC but were able to tolerate it but consumed more calories leaving less for growth and repair of damaged tissue leading to prolonged need for support.
Either way, the use of HFNC was found to lead to worse outcomes and in the ELBW infant should be avoided as routine practice pending the results of a prospective RCT on the subject.
Is it a total ban though?
As with many treatments that one should not consider standard of care there may be some situations where there may be benefit. The ELBW infant with nasal breakdown from CPAP that despite excellent nursing and RRT attention continues to demonstrate tissue damage is one patient that could be considered. The cosmetic implications and potential for surgical correction at a later date would be one reason to consider a trial of HFNC but only in the patient that was close to being able to come off CPAP. In the end I believe that if a ELBW infant needs non invasive pressure support then it should be with CPAP but as there saying goes there may be a right time and a place for even this modality.







 Another interesting finding was that the most common reason for treatment failure was criteria 1 (FiO2 > 40%) while intubation was higher for all infants but did not reach statistical significance. Curiously what did reach a significant difference was criteria #4 (18.4% urgent intubations in the CPAP group vs 5.6% in the HFNC group). You might be tempted to therefore ponder which is worse, a little O2 or being intubated but you need to recall the trial design which was set up to provide this kind of result. If you failed HFNC you were placed on CPAP whereas if you failed CPAP you were intubated. In the HFNC group, 78 infants were deemed to have failed but 28 of them were in fact “rescued by CPAP”. It therefore isn’t a fair comparison when it comes to urgent intubation since if you failed CPAP there wasn’t another option.
Another interesting finding was that the most common reason for treatment failure was criteria 1 (FiO2 > 40%) while intubation was higher for all infants but did not reach statistical significance. Curiously what did reach a significant difference was criteria #4 (18.4% urgent intubations in the CPAP group vs 5.6% in the HFNC group). You might be tempted to therefore ponder which is worse, a little O2 or being intubated but you need to recall the trial design which was set up to provide this kind of result. If you failed HFNC you were placed on CPAP whereas if you failed CPAP you were intubated. In the HFNC group, 78 infants were deemed to have failed but 28 of them were in fact “rescued by CPAP”. It therefore isn’t a fair comparison when it comes to urgent intubation since if you failed CPAP there wasn’t another option.