
by All Things Neonatal | Sep 6, 2018 | Neonatal, Neonatology, newborn, preemie
It has been a few months now that I have been serving as Chair of the Fetus and Newborn Committee for the Canadian Pediatric Society. Certain statements that we release resonate strongly with me and the one just released this week is certainly one of them. Guidelines for vitamin K prophylaxis in newborns is an important statement about a condition that thankfully so few people ever experience. To read the statement on the CPS website click here.
Similar story to vaccinations
Prior to the American Academy of Pediatrics in 1961 proclaiming that all newborns should receive IM Vitamin K at birth the incidence of Vitamin K deficient bleeding was 0.25 – 1.7%. Think about that for a moment. A new parent could expect that 1/100 babies roughly might have intestinal bleeding or worse an intracranial hemorrhage due to an insufficient amount of vitamin K levels in the newborn. The types of bleeding could be categorized into three different time epochs. Early onset (occurring in the first 24 hours post-birth), classic (occurring at days 2 to 7) and late onset (at 2 to 12 weeks and up to 6 months of age).
With a rate that high detractors of providing Vitamin K at birth would say “why should we give it; I haven’t heard of any baby getting such bleeding?” Looking at it another way though, why don’t you see congenital rubella or kids with measles much these days? It’s due to vaccination. Thankfully as a Neonatologist, I don’t see Vitamin K deficient bleeding since most parents provide Vitamin K to their babies at birth. If you went back to the era prior to 1961 when widespread supplementation of Vitamin K began in the US, I imagine it would not have been too uncommon to hear about a baby who had bleeding issues after birth. Just because we don’t hear about German Measles much anymore doesn’t mean the virus causing it doesn’t still exist!
How Effective is Vitamin K?
How effective is Vitamin K administration at birth in preventing hemorrhagic disease of the newborn (HDNB)? Studies estimate an incidence of 0.25 per 100000 live births or 1 in 400000 babies vs the 1/100 risk without any vitamin K. That is one effective intervention! At this point I would ask those families that are still concerned about giving Vitamin K to their infants if this is a risk they can accept? If they refuse Vitamin K and there is a significant bleed how will they react?
The Change in this CPS Statement From the Past
In the last statement on Vitamin K, the authors suggested that the oral route was a reasonable option. Instead of giving 1 mg of Vitamin K IM one would dose it as 2 mg orally and then repeat at 2-4 weeks and then 6-8 weeks. In looking at the effectiveness though it is worth noting that while we can assure that families will get the first dose, as with any medication that needs repeat dosing there is the risk of forgetfulness leading to missed dosing down the road. In fact when the authors looked at the risk of late HDNB they found the following “The relative risk for VKDB, when comparing PO versus IM vitamin K administration in these two studies, was 28.75 (95% CI 1.64 to 503.45) and 5.97 (95% CI 0.54 to 65.82), respectively [19][20].”
The outcome of course remains rare but the risk based on two studies was almost 30 times higher than if IM dosing was given.
On this basis IM is recommended.
Having said all this I recognize that despite all this information, some families will choose for a number of reasons to still opt for the oral dose. As the statement suggests we need to encourage such use when a family refuses IM vitamin K. The 30 fold risk compared to IM administration is magnitudes lower than the approximate 1/100 risk of giving nothing at all!
In the end I believe that one case of intracranial hemorrhage from inadequate vitamin K is too much. This one vitamin indeed could save a life.

by All Things Neonatal | Aug 15, 2018 | intubation, Neonatal, Neonatology, technology
The modern NICU is one that is full of patients on CPAP these days. As I have mentioned before, the opportunity to intubate is therefore becoming more and more rare is non-invasive pressure support becomes the mainstay of therapy. Even for those with established skills in placing an endotracheal tube, the number of times one gets to do this per year is certainly becoming fewer and fewer. Coming to the rescue is the promise of easier intubations by being able to visualize an airway on a screen using a video laryngoscope. The advantage to the user is that anyone who is watching can give you some great tips and armed with this knowledge you may be better able to determine how to adjust your approach.
For those of you who have followed the blog for some time, you will recall this is not the first time video laryngoscopy has come up. I have spoken about this before in Can Video Laryngoscopy Improve Trainee Success in Intubation. In that piece, the case was made that training residents how to intubate using a video laryngoscope (VL) improves their success rate. An additional question that one might ask though has to do with the quality of the intubation. What if you can place a tube using a video laryngoscope but the patient suffers in some way from having that piece of equipment in the mouth? Lucky for us some researchers from the Children’s Hospital of Philadelphia have completed a study that can help answer this additional question.
Video Laryngoscopy may work but does it cause more harm than good?
Using a video laryngoscope requires purchasing one first and they aren’t necessarily cheap. If they were to provide a better patient experience though the added cost might well be worth it. Pouppirt NR et al published Association Between Video Laryngoscopy and Adverse Tracheal Intubation-Associated Events in the Neonatal Care Unit. This study was a retrospective comparison of two groups; one having an intubation performed with a VL (n=161 or 20% of the group) and the other with a standard laryngoscope (644 or 80% of the group). The study relied on the use of the National Emergency Airway Registry for Neonates (NEAR4NEOs), which records all intubations from a number of centres using an online database and allows for analysis of many different aspects of intubations in neonates. In this case the data utilized though was from their centre only to minimize variation in premedication and practitioner experience.
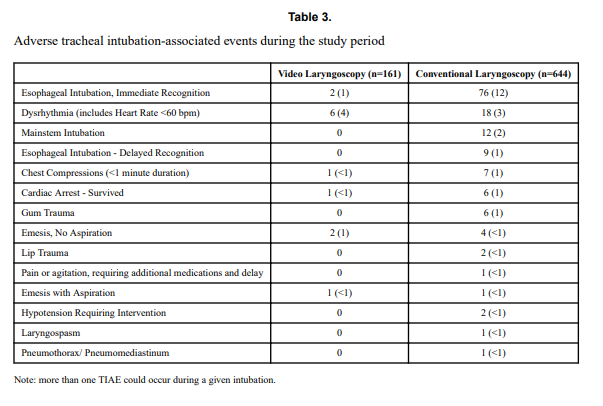
Tracheal intubation adverse events (TIAEs) were subdivided into severe (cardiac arrest, esophageal intubation with delayed recognition, emesis with witnessed aspiration, hypotension requiring intervention (fluid and/or vasopressors), laryngospasm, malignant hyperthermia, pneumothorax/pneumomediastinum, or direct airway injury) vs non-severe (mainstem bronchial intubation, esophageal intubation with immediate recognition, emesis without aspiration, hypertension requiring therapy, epistaxis, lip trauma, gum or oral trauma, dysrhythmia, and pain and/or agitation requiring additional medication and causing a delay in intubation.
Looking at the patient characteristics and outcomes, some interesting findings emerge.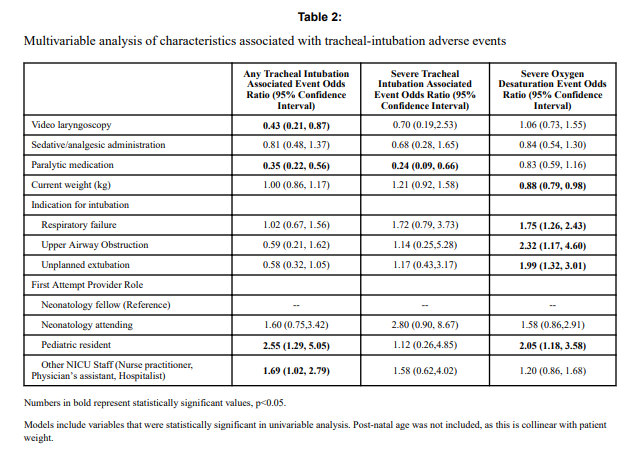

Patients who had the use of the VL were older and weighed more. They were more likely to have the VL used for airway obstruction than respiratory failure and importantly were also more likely to receive sedation/analgesia and paralysis. These researchers have also recently shown that the use of paralysis is associated with less TIAEs so one needs to bear this in mind when looking at the rates of TIAEs. There were a statistically significant difference in TIAEs of any type of 6% in the VL group to 19% in the traditional laryngoscopy arm but severe TIAEs showed not difference.
Given that several of the baseline characteristics might play a role in explaining why VL seemed superior in terms of minimizing risk of TIAEs by two thirds, the authors performed a multivariable analysis in which they took all factors that were different into account and then looked to see if there was still an effect of the VL despite these seemingly important differences. Interestingly, use of VL showed an Odds ratio of 0.43 (0.21,0.87 95% CI) in spite of these differences.
What does it mean?
Video laryngoscopy appears to make a difference to reducing the risk on TIAEs as an independent factor. The most common TIAE was esophageal intubation at 10% and reducing that is a good thing as it leads to fewer intubation attempts. This was also sen as the first attempt success was 63% in the VL group vs 44% in the other.
Now we need to acknowledge that this was not a randomized controlled trial so it could indeed be that there are other factors that the authors have not identified that led to improvements in TIAEs as well. What makes this study so robust though is the rigour with which the centre documents all of their intubations using such a detailed registry. By using one centre much of the variability in practice between units is eliminated so perhaps these results can be trusted. Would your centre achieve these same results? Maybe not but it would certainly be interesting to test drive one of these for a period of time see how it performs.
by All Things Neonatal | Jul 26, 2018 | Neonatal, Neonatology, resuscitation
It is hard to believe but it has been almost 3 years since I wrote a piece entitled A 200 year old invention that remains king of all tech in newborn resuscitation. In the post I shared a recent story of a situation in which the EKG leads told a different story that what our ears and fingers would want us to believe. The concept of the piece was that in the setting of pulseless electrical activity (where there is electrical conductance in the myocardium but lack of contraction leaves no blood flow to the body) one could pick up a signal from the EKG leads when there is in fact no pulse or perfusion to vital organs. This single experience led me to postulate that this situation may be more common than we think and the application of EKG leads routinely could lead to errors in decision making during resuscitation of the newborn. It is easy to see how that could occur when you think about the racing pulses of our own in such situations and once chest compressions start one might watch the monitor and forget when they see a heart rate of 70 BPM to check for a corresponding pulse or listen with the stethoscope. I could see for example someone stopping chest compressions and continuing to provide BVM ventilation despite no palpable pulse when they see the QRS complex clearly on the monitor. I didn’t really have much evidence to support this concern but perhaps there is a little more to present now.
A Crafty Animal Study Provides The Evidence
I haven’t presented many animal studies but this one is fairly simple and serves to illustrate the concern in a research model. For those of you who haven’t done animal research, my apologies in advance as you read what happened to this group of piglets. Although it may sound awful, the study has demonstrated that the concern I and others have has is real.
For this study 54 newborn piglets (equivalent to 36-38 weeks GA in humans) were anesthetized and had a flow sensor surgically placed around the carotid artery. ECG leads were placed as well and then after achieving stabilization, hypoxia was induced with an FiO2 of 0.1 and then asphyxia by disconnecting the ventilator and clamping the ETT. By having a flow probe around the carotid artery the researchers were able to determine the point of no cardiac output and simultaneously monitor for electrical activity via the EKG leads. Auscultation for heart sounds was performed as well.
The results essentially confirm why I have been concerned with an over reliance on EKG leads.

Of the 57 piglets, 14 had asystole and no carotid flow but in 23 there was still a heart rate present on the EKG with no detectable carotid flow. This yields a sensitivity of only 37%. Moreover, the overall accuracy of the ECG was only 56%.
Meanwhile the stethoscope which I have referred to previously as the “king” in these situations had 100% sensitivity so remains deserving of that title.
What do we do with such information?
I think the results give us reason to pause and remember that faster isn’t always better. Previous research has shown that signal acquisition with EKG leads is faster than with oximetry. While a low heart rate detected quickly is helpful to know what the state of the infant is and begin the NRP pathway, we simply can’t rely on the EKG to tell us the whole story. We work in interdisciplinary teams and need to support one another in resuscitations and provide the team with the necessary information to perform well. The next time you are in such a situation remember that the EKG is only one part of the story and that auscultation for heart sounds and palpation of the umbilical cord for pulsation are necessary steps to demonstrate conclusively that you don’t just have a rhythm but a perfusing one.
I would like to thank the Edmonton group for continuing to put out such important work in the field of resuscitation!

by All Things Neonatal | Jul 9, 2018 | hemodynamics
Welcome to the home page for our Integrated Evaluation of Hemodynamics program at the University of Manitoba. This program began in Winnipeg, Manitoba, Canada in 2014 and has been growing ever since.
What is considered normal hemodynamics?
1. Intact or normal hemodynamics implies blood flow that provides adequate oxygen and nutrient delivery to the tissues.
2. Blood flow varies with vascular resistance and cardiac function; both may be reflected in blood pressure(2). Normal cardiovascular dynamics should be considered within the context of global hemodynamic function, with the aim of achieving normal oxygen delivery and end organ performance
3. The current routine assessment of hemodynamics in sick preterm and term infants is based on incomplete information. We have addressed this by adopting an approach utilizing objective techniques, namely integrating targeted neonatal echocardiography (TNE) with near-infrared spectroscopy (NIRS). Implementation of these techniques requires an individual with the requisite TNE training, preferably in an accredited program, who also has a good understanding of perinatal and neonatal cardiovascular, respiratory, and other specific end organ physiology.
Why are premature infants more susceptible to cardiovascular compromise?
Hemodynamic compromise in the early neonatal period is common and may lead to unfavorable neurodevelopmental outcome4. A thorough understanding of the physiology of the cardiovascular system in the preterm infants, influence of antenatal factors, and postnatal adaptation is essential for the management of these infants during the early critical phase5. The impact of the various ventilator modes, the presence of a patent ductus arteriosus (PDA), and systemic inflammation all may affect the hemodynamics6. The poor clinical indicators of systemic perfusion and the relative insensitivity of conventional echocardiographic techniques in assessing myocardial contractility mean that monitoring of the hemodynamics of the preterm infant remains a challenge7.
What is integrated hemodynamics in neonatal care?
Integrated hemodynamics focuses on how to interpret multiple tools of hemodynamics evaluation in sick infants (TNE, clinical details, NIRS, organ specific ultrasound) and the art of formulating a pathophysiologic relevant medical recommendation.
Main objectives of applying Targeted Neonatal Echocardiography and Evaluation of Neonatal hemodynamics
Optimise care of infants with hemodynamic compromise to prevent progression into late irreversible stages of shock (Hypoxia)
Decrease overall PDA related complications (Hypoxemia and hypoxia)
Optimize care of infants with hypoxemic respiratory failure (HRF)
Decrease the incidence of progression of infants with hypoxemic respiratory failure and shock to end organ dysfunction
Objective of the program
Orientation to the hemodynamics concepts and basics
Orientation to the 3 level of the pathophysiologic approach to hemodynamics:
Level one: Relying on blood pressure trends (systole, diastole, and pulse pressure) and waveforms with other clinical parameters (all NICU practitioners)
Level one plus (advanced monitoring): Relying on blood pressure trend and near infrared spectroscopy (NIRS) for assessment of hemodynamics and oxygen extraction (optional to NICU practitioners)
Level two (TNE approach): Relying on both clinical parameters and TNE for objective assessment of cardiac output, extra and intra cardiac shunts, systemic and pulmonary vascular resistance. (Neonatologist trained on TNE)
Level three (integrated evaluation of hemodynamics): integrating blood pressure trends, TNE and NIRS for assessment of oxygen delivery, specific end organ oxygen consumption and the degree of compensation (comprehensive hemodynamic approach)
Understanding the rationale for the measurements and the specific values for each disease, and recognize limitations of the 3 models
To see research that we have done in the area of Integrated Hemodynamics please see our publication list that can be found here.
To access our video series providing examples of TNE and presentations on the use of hemodynamics in clinical application please see our Youtube channel playlist “Integrated Neonatal Hemodynamics”
References
Wolff CB. Normal cardiac output, oxygen delivery and oxygen extraction. Adv Exp Med Biol. 2008;599:169-182. doi:10.1007/978-0-387-71764-7-23.
Azhan A, Wong FY. Challenges in understanding the impact of blood pressure management on cerebral oxygenation in the preterm brain. Front Physiol. 2012;3 DEC(December):1-8. doi:10.3389/fphys.2012.00471.
de Boode WP. Clinical monitoring of systemic hemodynamics in critically ill newborns. Early Hum Dev. 2010;86(3):137-141. doi:10.1016/j.earlhumdev.2010.01.031.
Sehgal A. Haemodynamically unstable preterm infant: an unresolved management conundrum. Eur J Pediatr. 2011;170(10):1237-1245. doi:10.1007/s00431-011-1435-4.
Vutskits L. Cerebral blood flow in the neonate. Paediatr Anaesth. 2014;24(2):22-29. doi:10.1111/pan.12307.
Noori S, Stavroudis T a, Seri I. Systemic and cerebral hemodynamics during the transitional period after premature birth. Clin Perinatol. 2009;36(4):723-36, v. doi:10.1016/j.clp.2009.07.015.
Elsayed YN, Amer R, Seshia MM. The impact of integrated evaluation of hemodynamics using targeted neonatal echocardiography with indices of tissue oxygenation: a new approach. J Perinatol. 2017. doi:10.1038/jp.2016.257.

by All Things Neonatal | Jun 30, 2018 | intubation, Neonatal, Neonatology, newborn, preemie, Prematurity
A few weeks back I wrote about the topic of intubations and whether premedication is really needed (Still performing awake intubations in newborns? Maybe this will change your mind.) I was clear in my belief that it is and offered reasons why. There is another group of practitioners though that generally agree that premedication is beneficial but have a different question. Many believe that analgesia or sedation is needed but question the need for paralysis. The usual argument is that if the intubation doesn’t go well and the patient can’t spontaneously ventilate could we be worse off if the patient loses their muscle tone.
Neonatal Intubation Registry
At the CPS meeting last month in Quebec City. I had the pleasure of listening to a talk by Dr. Elizabeth Foglia on the findings from a Neonatal intubation registry that many centres have been contributing to. The National Emergency Airway Registry for Neonates (NEAR4NEOs), records all intubations from a number of centres using an online database and allows for analysis of many different aspects of intubations in neonates.
This year, J. Krick et al published Premedication with paralysis improves intubation success and decreases adverse events in very low birth weight infants: a prospective cohort study. This study compared results from the registry of two centres, the University of Washington Medical Center (UWMC) and Seattle Children’s Hospital where the former rarely uses paralysis and the latter in almost all instances of non-emergent intubation. In all, 237 encounters were analyzed in the NICU for babies < 1500g with the majority of encounters (181) being from UWMC. The median PMA at intubation was 28 completed weeks (IQR: 27, 30), chronological age was 9 days (IQR: 2, 26) and weight was 953 g (IQR: 742,1200). The babies were compared based on the following groups. Premedication with a paralytic 21%, without a paralytic 46% and no premedication 31%.
This was an observational study that examined the rates of adverse events and subdivided into severe (cardiac arrest, esophageal intubation with delayed recognition, emesis with witnessed aspiration, hypotension requiring intervention (fluid and/or vasopressors), laryngospasm, malignant hyperthermia, pneumothorax/pneumomediastinum, or direct airway injury) vs non-severe (mainstem bronchial intuba- tion, esophageal intubation with immediate recognition, emesis without aspiration, hypertension requiring therapy, epistaxis, lip trauma, gum or oral trauma, dysrhythmia, and pain and/or agitation requiring additional medication and causing a delay in intubation.).
How did the groups compare?
It turns out paralysis seems to be a big deal (at least in this group of infants). Use of paralysis resulted in less attempts to intubate (median 1 attempt; IQR: 1, 2.25 vs. 2; IQR: 1, 3, p < 0.05)). In fact success was no different between the groups with no paralysis or no premedication at all! When it comes to tracheal intubation adverse events the impact of using paralysis becomes more evident. 
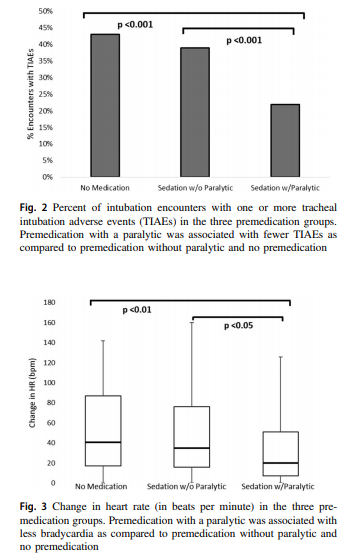 Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.
Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.
How do we interpret the results?
Based on the results from the registry it looks like paralysis is a good thing here when electively intubating infants. If we try to determine the reason for it I suspect it may have much to do with the higher likelihood of success on the first attempt at placing an ETT. The longer it takes to place the ETT or the more number of attempts requiring intermittent PPV in a patient who truly needs a tube the greater the likelihood that you will see adverse events including bradycardia. It may simply be that a calm and still patient is an easier intubation and getting the tube in faster yields a more stable patient.
I am biased though and I think it is worth pointing out another possible reason for the differing results. One hospital in this study routinely used premedication and the other did not. Almost 3/4 of the patients came from one hospital which raises the possibility that skill set could be playing a role. If the skill of providers at the two hospitals differed, the results could reflect the variable skill in the practitioners versus the difference in the medications used themselves. What I don’t know though is whether the two share the same training program or not. Are the trainees the same at both sites (google maps says the two sites are 11 minutes away by car)? The difference still might be in local respiratory therapists or Neonatologists intubating as well. Regardless, the study provides evidence that paralysis makes a difference. To convince those out there though who remain skeptical I think we are going to need the registry to take part in a prospective trial using many centres. A format in which several centres that don’t use paralysis are compared to several who do routinely would help to sort out the concern in skill when looking only at two centres. This wouldn’t be randomized of course but I think it would be very difficult at this point to get a centre that strongly believes in using paralysis to randomize so a prospective study using groups chosen by the individual centre might be the next best thing. If anyone using the registry is reading this let me know what you think?










 Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.
Paralysis does make a difference in reducing the incidence of such events and moreover when only looking at the rate of severe adverse events as defined above the finding was that none occurred when paralysis was used vs 9 when no paralysis was employed and 5 when no premedication was used at all. The rate of bradycardic events was less in the paralytic group but rates of oxygen desaturation between the three arms were no different.