by All Things Neonatal | Oct 3, 2018 | kangaroo care
By Diane Schultz
A Mother’s arms are full of tenderness and children sleep soundly in them – Victor Hugo
The NICU is a loud and chaotic place, that can be painful to be in at times. Its hard to get a good nights sleep (especially for the nurses!). When you think about how much our infants are handled and disturbed, poked and prodded, all in almost continual daylight, it’s a wonder they get any sleep.
For normal neurodevelopment the infant needs both active and quiet sleep. Sleep in an infant is divided into REM (active sleep) and NON-REM (quiet sleep). During quiet sleep you see very little movement and a regular breathing pattern, whereas active sleep involves movement with an irregular breathing pattern.
The importance of Quiet Sleep:
• Without it, the infant doesn’t get enough active sleep.
• Provides the infant with a break from the busy NICU environment.
• Lessons the release of glucocorticoids (Increased cortisol can cause neuronal cell death).
• Restorative.
• Necessary for brain development.
• Increased quiet sleep = decreased risk of SIDS.
The importance of Active Sleep:
• Active sleep promotes brain maturation (US DHHS, 2003; Mirmiran, 1995).
• Most memory consolidation and learning occurs in this state (Smith, 2003).
• Nerve cell connections are restructured (synaptic plasticity) (Marks et al., 1995).
Due to the NICU environment, the infant ends up having slower sleep organization maturation and with increased cortisol they are more apt to have a disturbed and less restful sleep.
A complete cycle of sleep includes moving from active sleep to quiet sleep and back to active sleep. Full term and preterms >32 weeks postconceptional age will need about 60-70 minutes for a cycle. Infants <32 weeks postconceptional age will need about 90 minutes. So when infants come out for KC, we try to plan for at least that amount of time.
You will see when infants are placed in KC, the infant settles and goes into a deep sleep. To accommodate this, you will need comfortable chairs for the parent and good support for their arms. You also want to make sure they have had something to eat or drink, pumped breast milk, used the washroom and had something for pain if needed. Don’t be surprised if your parent falls asleep as well; oxytocin will end up kicking in (the cuddle hormone) and they often find it hard to stay awake. We also provide warmed blankets for our parents to encourage everyone to get comfortable and rest. Snoring is a common side effect of KC in our unit…
While in KC, the infants have a deep sleep with less arousal and better sleep organization than when not in KC (Ludington-Hoe et al., 2006)
In Scher et al.’s study (2009) they found that infants’ brain maturation was accelerated and brain complexity increased with 1.5 hours of KC/day for 4 days/wk from 32-40wks pma. Enhanced development in five sensory areas of the brain was shown with KC that was not seen in infants who did not get KC (both preterm and full term).
With all the evidence pointing to KC being beneficial for a good night’s sleep, I find it difficult to understand why so many are skeptical of it!
Sleep is that golden chain that ties health and our bodies together – Thomas Dekker
by All Things Neonatal | Sep 20, 2018 | resuscitation
One of the first things a student of any discipline caring for newborns is how to calculate the apgar score at birth. Over 60 years ago Virginia Apgar created this score as a means of giving care providers a consistent snapshot of what an infant was like in the first minute then fifth and if needed 10, 15 and so on if resuscitation was ongoing. For sure it has served a useful purpose as an apgar score of 0 and 0 gives one cause for real worry. What about a baby with an apgar of 3 and 7 or 4 and 8? There are certainly infants who have done very well who initially had low apgar scores and conversely those who had higher apgar scores who have had very significant deleterious outcomes including death. I don’t mean to suggest that the apgar scores don’t provide any useful predictive value as they are used as part of the criteria to determine if a baby merits whole body cooling or not. The question is though after 60+ years, has another score been created to provide similar information but enhance the predictive value derived from a score?
The Neonatal Resuscitation and Adaptation Score (NRAS)
Back in 2015 Jurdi et al published Evaluation of a Comprehensive Delivery Room Neonatal Resuscitation and Adaptation Score (NRAS) Compared to the Apgar Score. This new score added into a ten point score resuscitative actions taken at the 1 and 5 minute time points to create a more functional score that included interventions. The other thing this new score addressed was more recent data that indicated a blue baby at birth is normal (which is why we have eliminated asking the question “is the baby pink?” in NRP. Knowing that, the colour of the baby in the apgar score may not really be that relevant. Take for example a baby with an apgar score of 3 at one minute who could have a HR over 100 and be limp, blue and with shallow breathing. Such a baby might get a few positive pressure breaths and then within 10 seconds be breathing quite well and crying. Conversely, they might be getting ongoing PPV for several minutes and need oxygen. Were they also getting chest compressions? If I only told you the apgar score you wouldn’t have much to go on. Now look at the NRAS and compare the information gathered using two cardiovascular (C1&2), one neurological test (N1) and two respiratory assessments (R1&2).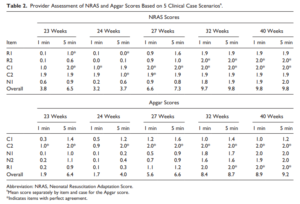

The authors in this study performed a pilot study on only on 17 patients really as a proof of concept that the score could be taught and implemented. Providers reported both scores and found “superior interrater reliability (P < .001) and respiratory component reliability (P < .001) for all gestational ages compared to the Apgar score.”
A Bigger Study Was Needed
The same group in 2018 this time led by Witcher published Neonatal Resuscitation and Adaptation Score vs Apgar: newborn assessment and predictive ability. The primary outcome was the ability of a low score to predict mortality with a study design that was a non-inferiority trial. All attended deliveries were meant to have both scores done but due to limited numbers of trained personnel who could appropriately administer both scores just under 90% of the total deliveries were assigned scores for comparison. The authors sought to recruit 450 infants to show that a low NRAS score (0–3) would not be inferior to a similar Apgar at predicting death. Interestingly an interim analysis found the NRAS to be superior to Apgar when 75.5% of the 450 were enrolled, so the study was stopped. What led the apgar score to perform poorly in predicting mortality (there were only 12 deaths though in the cohort) was the fact that 49 patients with a 1 minute apgar score of 0-3 survived compared to only 7 infants with a low NRAS score.
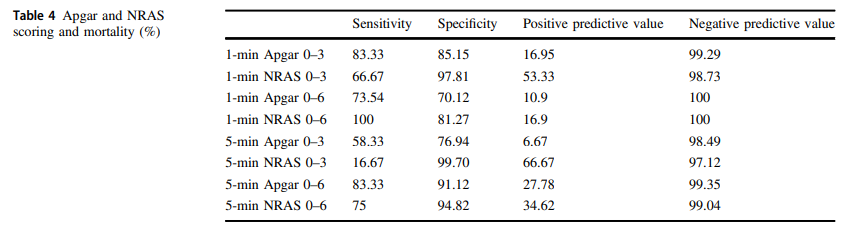
The other interesting finding was the ability of the NRAS to predict the need for respiratory support at 48 hours with a one minute apgar score of 0-3 being found in 39% of those on support compared to 100% of those with a low NRAS. Also at 5 minutes a score of 4-6 for the apgar was found in 48% of those with respiratory support at 48 hours vs 87% of those with a similar range NRAS. These findings were statistically significant while a host of other conditions such as sepsis, hypoglycemia, hypothermia and others were no different in terms of predictive ability of the scores.
An Even Bigger Study is Needed
To be sure, this study is still small and missed just over 90% of all deliveries so it is possible there is some bias that is not being detected here. I do think there is something here though which a bigger study that has an army of people equipped to provide the scoring will add to this ongoing story. Every practitioner who resuscitates an infant is asked at some point in those first minutes to hour “will my baby be ok?”. The truth is that the apgar score has never lived up to the hope that it would help us provide an accurate clairvoyant picture of what lies ahead for an infant. Where this score gives me hope is that a score which would at the very least help me predict whether an infant would likely still be needing respiratory support in 48 hours provides the basic answer to the most common question we get in the unit once admitted; “when can I take my baby home”. Using this score I could respond with some greater confidence in saying “I think your infant will be on support for at least 48 hours”. The bigger question though which thankfully we don’t have to address too often for the sickest babies at birth is “will my baby survive?”. If a larger study demonstrates this score to provide a greater degree of accuracy then the “Tipping Point” might just be that to switching over to the NRAS and leaving the apgar score behind. That will never happen overnight but medicine is always evolving and with time you the reader may find yourself becoming very familiar with this score!

by All Things Neonatal | Sep 6, 2018 | Neonatal, Neonatology, newborn, preemie
It has been a few months now that I have been serving as Chair of the Fetus and Newborn Committee for the Canadian Pediatric Society. Certain statements that we release resonate strongly with me and the one just released this week is certainly one of them. Guidelines for vitamin K prophylaxis in newborns is an important statement about a condition that thankfully so few people ever experience. To read the statement on the CPS website click here.
Similar story to vaccinations
Prior to the American Academy of Pediatrics in 1961 proclaiming that all newborns should receive IM Vitamin K at birth the incidence of Vitamin K deficient bleeding was 0.25 – 1.7%. Think about that for a moment. A new parent could expect that 1/100 babies roughly might have intestinal bleeding or worse an intracranial hemorrhage due to an insufficient amount of vitamin K levels in the newborn. The types of bleeding could be categorized into three different time epochs. Early onset (occurring in the first 24 hours post-birth), classic (occurring at days 2 to 7) and late onset (at 2 to 12 weeks and up to 6 months of age).
With a rate that high detractors of providing Vitamin K at birth would say “why should we give it; I haven’t heard of any baby getting such bleeding?” Looking at it another way though, why don’t you see congenital rubella or kids with measles much these days? It’s due to vaccination. Thankfully as a Neonatologist, I don’t see Vitamin K deficient bleeding since most parents provide Vitamin K to their babies at birth. If you went back to the era prior to 1961 when widespread supplementation of Vitamin K began in the US, I imagine it would not have been too uncommon to hear about a baby who had bleeding issues after birth. Just because we don’t hear about German Measles much anymore doesn’t mean the virus causing it doesn’t still exist!
How Effective is Vitamin K?
How effective is Vitamin K administration at birth in preventing hemorrhagic disease of the newborn (HDNB)? Studies estimate an incidence of 0.25 per 100000 live births or 1 in 400000 babies vs the 1/100 risk without any vitamin K. That is one effective intervention! At this point I would ask those families that are still concerned about giving Vitamin K to their infants if this is a risk they can accept? If they refuse Vitamin K and there is a significant bleed how will they react?
The Change in this CPS Statement From the Past
In the last statement on Vitamin K, the authors suggested that the oral route was a reasonable option. Instead of giving 1 mg of Vitamin K IM one would dose it as 2 mg orally and then repeat at 2-4 weeks and then 6-8 weeks. In looking at the effectiveness though it is worth noting that while we can assure that families will get the first dose, as with any medication that needs repeat dosing there is the risk of forgetfulness leading to missed dosing down the road. In fact when the authors looked at the risk of late HDNB they found the following “The relative risk for VKDB, when comparing PO versus IM vitamin K administration in these two studies, was 28.75 (95% CI 1.64 to 503.45) and 5.97 (95% CI 0.54 to 65.82), respectively [19][20].”
The outcome of course remains rare but the risk based on two studies was almost 30 times higher than if IM dosing was given.
On this basis IM is recommended.
Having said all this I recognize that despite all this information, some families will choose for a number of reasons to still opt for the oral dose. As the statement suggests we need to encourage such use when a family refuses IM vitamin K. The 30 fold risk compared to IM administration is magnitudes lower than the approximate 1/100 risk of giving nothing at all!
In the end I believe that one case of intracranial hemorrhage from inadequate vitamin K is too much. This one vitamin indeed could save a life.

by All Things Neonatal | Aug 8, 2018 | kangaroo care, skin to skin, Uncategorized
By Diane Schultz
I thought I would start off my series of posts with one of the most basic reasons we do Kangaroo Care.
Thermoregulation is the process of maintaining an infant’s temperature within normal range. Thermoregulation is extremely important for the newborn (term or preterm). An infant’s body surface area is 3X greater than an adult’s, causing them to potentially lose heat rapidly, up to 4X faster. When cold stressed, infants use energy and oxygen to generate warmth. Oxygen consumption can increase by as much as 10%. Thermoregulation of the infants allows them to conserve energy and build up *reserves”.
What Happens When An infant Is Placed Skin to Skin?
When the term infant is placed skin to skin at birth, the mother’s breasts immediately start to warm and conduct heat to the infant, helping to maintain normal blood sugar levels due to the infant not having to use their own brown fat to stay warm (Bergstrom et al.,2007;Bystrova et al.,2007;Ludington-Hoe et al.,2000,2006) (Chantry,2005;Christensson et al.,1992).
Kangaroo Care maintains a Neutral Thermal Environment (defined as the ideal setting in which an infant can maintain a normal body temperature while producing only the minimum amount of heat generated from basal life-sustaining metabolic processes).
In our unit, any infant that needs an incubator to maintain their temperature can only come out to be held by Kangaroo Care instead of being bundle held. To help maintain thermoregulation we make sure the infant and parent are in a draft free area, and use 2-4 layers of blankets over the infant (you can always remove a layer if needed). Infants weighing less than 1000gms should wear some type of head cap and monitor them using the incubator’s temperature probe. Remember too, we don’t want any bras or clothing between the infant and the mother, fabric will interfere with the conductance of heat from mother to infant (Ludington-Hoe et al.,2000).
One of the interesting things about KC and thermoregulation is if a mother holds twins in KC each breast works independently to warm each infant (Ludington-Hoe, et al.,2006). Triplets? Not sure, but our mothers hold their “trips” together all the time and we have had no issues.
Now, how about the father? Does he thermoregulate like the mother? With mothers you have what is called Thermal Synchrony (maternal breast temperatures changing in response to the infant’s temperature) (Ludington-Hoe, et al.,1990;1994,2000) where the fathers chests will warm up when the infant is placed in KC but will not cool down (Maastrup & Greisen, 2010). We don’t have any issues with our fathers overheating, just lots of hair to be picked off the infant after!
by All Things Neonatal | Jul 26, 2018 | Neonatal, Neonatology, resuscitation
It is hard to believe but it has been almost 3 years since I wrote a piece entitled A 200 year old invention that remains king of all tech in newborn resuscitation. In the post I shared a recent story of a situation in which the EKG leads told a different story that what our ears and fingers would want us to believe. The concept of the piece was that in the setting of pulseless electrical activity (where there is electrical conductance in the myocardium but lack of contraction leaves no blood flow to the body) one could pick up a signal from the EKG leads when there is in fact no pulse or perfusion to vital organs. This single experience led me to postulate that this situation may be more common than we think and the application of EKG leads routinely could lead to errors in decision making during resuscitation of the newborn. It is easy to see how that could occur when you think about the racing pulses of our own in such situations and once chest compressions start one might watch the monitor and forget when they see a heart rate of 70 BPM to check for a corresponding pulse or listen with the stethoscope. I could see for example someone stopping chest compressions and continuing to provide BVM ventilation despite no palpable pulse when they see the QRS complex clearly on the monitor. I didn’t really have much evidence to support this concern but perhaps there is a little more to present now.
A Crafty Animal Study Provides The Evidence
I haven’t presented many animal studies but this one is fairly simple and serves to illustrate the concern in a research model. For those of you who haven’t done animal research, my apologies in advance as you read what happened to this group of piglets. Although it may sound awful, the study has demonstrated that the concern I and others have has is real.
For this study 54 newborn piglets (equivalent to 36-38 weeks GA in humans) were anesthetized and had a flow sensor surgically placed around the carotid artery. ECG leads were placed as well and then after achieving stabilization, hypoxia was induced with an FiO2 of 0.1 and then asphyxia by disconnecting the ventilator and clamping the ETT. By having a flow probe around the carotid artery the researchers were able to determine the point of no cardiac output and simultaneously monitor for electrical activity via the EKG leads. Auscultation for heart sounds was performed as well.
The results essentially confirm why I have been concerned with an over reliance on EKG leads.

Of the 57 piglets, 14 had asystole and no carotid flow but in 23 there was still a heart rate present on the EKG with no detectable carotid flow. This yields a sensitivity of only 37%. Moreover, the overall accuracy of the ECG was only 56%.
Meanwhile the stethoscope which I have referred to previously as the “king” in these situations had 100% sensitivity so remains deserving of that title.
What do we do with such information?
I think the results give us reason to pause and remember that faster isn’t always better. Previous research has shown that signal acquisition with EKG leads is faster than with oximetry. While a low heart rate detected quickly is helpful to know what the state of the infant is and begin the NRP pathway, we simply can’t rely on the EKG to tell us the whole story. We work in interdisciplinary teams and need to support one another in resuscitations and provide the team with the necessary information to perform well. The next time you are in such a situation remember that the EKG is only one part of the story and that auscultation for heart sounds and palpation of the umbilical cord for pulsation are necessary steps to demonstrate conclusively that you don’t just have a rhythm but a perfusing one.
I would like to thank the Edmonton group for continuing to put out such important work in the field of resuscitation!








