.
It’s been a while since my last post. Like many centers across North America and worldwide the resuscitation of premature infants as young as 22 weeks is becoming more commonplace. Our own center is in the process of working towards coming up with evidence-based approaches to the care of these fragile infants. One of the questions that has long been asked is whether antenatal steroids really make a difference at these earliest gestational ages. The argument against effectiveness would be that the cards are just so stacked up against these preemies that even steroids may not help. Making matters worse is that the number of babies at this early gestational age included in antenatal steroid trials are extremely small making any conclusions difficult.
A Study To Help Us?
You can imagine my delight and then when I saw the following study published this past week, Association of Antenatal Steroid Exposure at 21 to 22 Weeks of Gestation With Neonatal Survival and Survival Without Morbidities.
In short, the goal of the study was to look at survival and survival without major morbidities for infant born between 22 and 0 days to 23 weeks and 6 days gestational age who either received no antenatal steroids, 1 dose or 2 doses 24 hours apart. Only those mothers who received betamethasone were included and the doses were provided at either 21 or 22 weeks of gestation prior to delivery at 22 and 23 weeks of gestation.
The study was retrospective and looked at NICHD neonatal research network data from January 1, 2016 to December 31, 2019. In comparison to all the previous prospective studies in existence which recruited less than 50 preterm infants this young this study managed to recruit 431 infants. In the groups analyzed, there were 25.5% infants who received no antenatal steroids, 18.6% infants receiving a partial course and 55.9% infants receiving complete antenatal steroids.
What did they find?
The authors found evidence that I believe will be reassuring to practitioners deciding whether to provide a course of steroids at these gestational ages. There are questions though that will be raised when looking at this data as well.
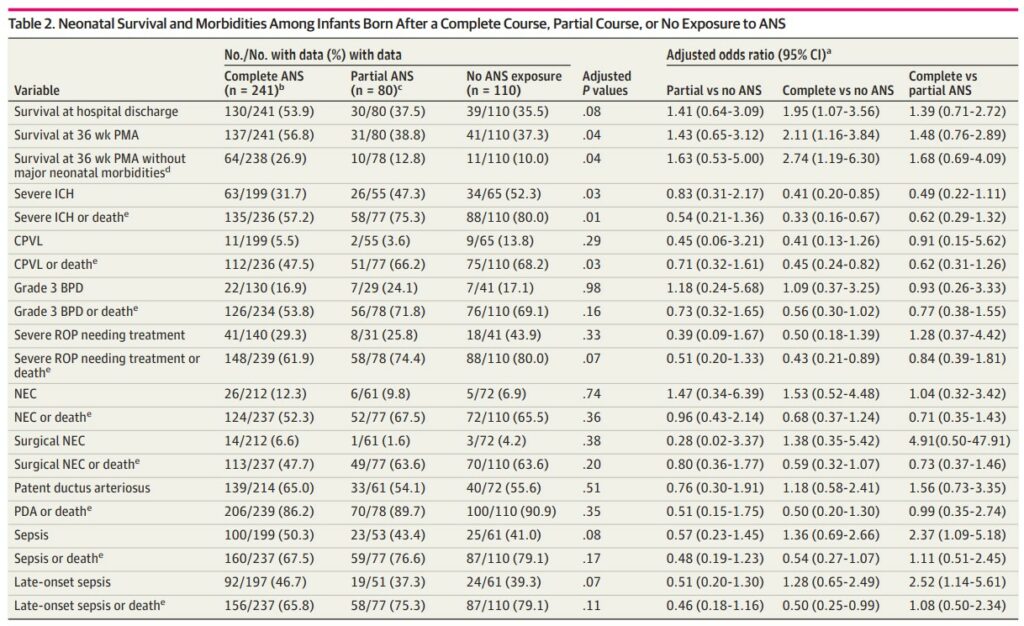
The data in table to show a number of interesting findings. Most notably a primary outcome of survival at hospital discharge was improved with a complete course of steroids but not with partial or none. Similarly there were reductions in severe intracranial hemorrhage and survival at 36 weeks postmenstrual age without major medical morbidities.
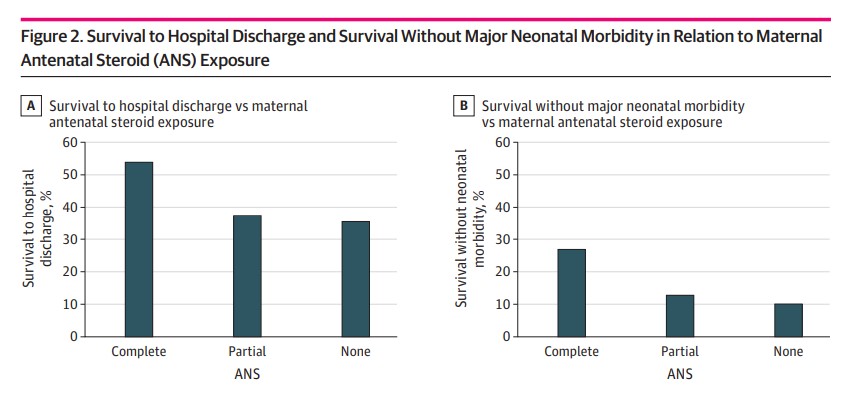
Figure 2 shows survival to hospital discharge and survival without major neonatal morbidities graphically. What one can more clearly see is that if you are going to give steroids the outcome is best if the mother receives both doses.
Challenges
On the one hand you might say that this is a slam dunk finding and we should be giving antenatal steroids to all women presenting at 21 and 22 weeks gestational age. I mentioned there would be questions and one of them will have to do with the avoidance of a repeat course of antenatal steroids. There is some literature that suggests repeat dosing of antenatal steroids later in pregnancy is associated with adverse developmental outcomes and also structural changes to the developing brain. This then leads the practitioner and a bit of a quagmire. If the woman presents at 21 or 22 weeks with threatened preterm labor do give her the steroids knowing that only a full course will help her versus waiting to see if she is truly in labor as you are considering whether you should save dosing for a later time in pregnancy. I have no doubt there will be some providers that we will hesitate to give the 1 course if that is their institution practice at this gestational age. This will not be an easy selection to make.
The other question that we will come up as we start to see a single dose antenatal steroid trials coming out is whether such infants will be included in prospective trials. The upcoming SNACS trial which we are participating in is one such trial that will include infants as young as these. It will be interesting to see if prospectively collected clinical trials with adequate numbers of such small infants will demonstrate similar findings that 2 doses really are required to make a meaningful reduction in adverse outcomes. As we have seen with many retrospective studies though such as this one the outcomes may in fact be different when you randomize patients in a prospective fashion.
For now I think the evidence as good as it is we will favor giving steroids to mother’s presenting at these gestational ages. Curious what you think?

