A Strategy to Minimize Blood Sampling in ventilated premature and term infants
As those of you who have been following this blog are aware, I am always on the lookout for strategies that can help minimize blood work without sacrificing care in the NICU. At particular risk our the very premature infants in our units who for example at 1 kg have about 80-90 mL of blood. It does not take very many 0.5 – 1 mL “small” draws to create anemia. In a recent study (free article in link) of infants less than 1500g entitled A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia, the authors studied 26 infants over a one month period. The results were staggering in that these infants experienced 138 +/- 21 blood draws with an average of nearly four transfusions per patient. While the authors do not specify what type of testing was done they did find a shocking statistic that 59% of the blood collected by weight of sample was discarded. This certainly stresses the point that we should aim to minimize the volume of sample collected in each case to that which is only necessary for the equipment to run. Furthermore, strategies to minimize sample draws should be utilized where possible and if accuracy permits point of care technology may further reduce volumes required and provide immediate results at the bedside. Lastly where possible, utilizing non-invasive technology to avoid blood draws needs to be explored when possible and was the subject of another post on Masimo non-invasive HgB measurement (http://wp.me/p5NWfD-1t).
Certainly in sick neonates whether they be term or preterm the drawing of blood gases to monitor ventilation contributes to the anemia of prematurity which often culminates in a transfusion. Sicker infants with greater lability due to respiratory compromise are deserving of optimal ventilation and this is achieved by monitoring pCO2 levels in arterial or venous samples. There have been different strategies employed to replace the sampling of CO2 by blood gas analysis which have not been very successful but there is one that I believe has promise that I will discuss at the end.
Transcutaneous pCO2 measurement was introduced in the 1980s. While this technology does allow measurement of pCO2 the variation between true arterial pCO2 and tcPCO2 can be wide making the technology difficult to implement on a consistent basis. In particular the accuracy in infants <28 weeks has been quite poor leading to increased numbers of arterial and venous samples to “check” ow closely the results correlate. As was described in 2005 by Aliwalas LL et al the technology in this group who actually have the highest number of blood draws does not meet the required standard to replace arterial pCO2 measurements (http://www.ncbi.nlm.nih.gov/pubmed/15496874)
Another method is of directly sampling exhaled CO2 in ventilated patients. Traditionally such measurements were taken with proximal gas sampling and in neonates in particular the results were discouraging. Problems encountered with proximal end tidal sampling were related to the lack of cuffed endotracheal tubes in part as the measured gas would be diluted with air in the presence of any leak around the tube leading to underestimation of true CO2 levels. Furthermore, in the presence of significant pulmonary disease the clearance of CO2 may be impaired such that the arterial pCO2 – ETCO2 difference may be quite large. For a review see the free article by Malloy and Deakins Are carbon dioxide detectors useful in neonates? The agreement between arterial and proximal sampling measured in this way has been quite variable and as such the technology has not really caught on to any great degree for monitoring ventilated infants. That being said it can be quite useful at determining if the endotracheal tube is in the trachea or esophagus. The presence of the waveform even if not yielding an accurate level confirms proper placement although where the tube sits in the trachea still needs confirmation.
The final method for sampling CO2 is the one which I believe holds the most promise for actually reducing blood draws and by extension risk of anemia and pain in the neonate. Kugelman and colleagues in Haifa, Israel published the following paper (free article in the link) A novel method of distal end-tidal CO2 capnography in intubated infants- comparison with arterial CO2 and with proximal mainstream end-tidal CO2. This creative study utilized a double lumen endotracheal tube which had been designed for surfactant installation and distal pressure measurement to instead sample pCO2 near the carina. This strategy was postulated to eliminate the issue with dilution of gas from proximal sampling and provide a closer measurement of true pCO2 when compared to arterial CO2 and proximal sampling. They studied 27 infants with varying degrees of pulmonary condition severity although most had RDS. When comparing the three methods of pCO2 measurement the following was found.
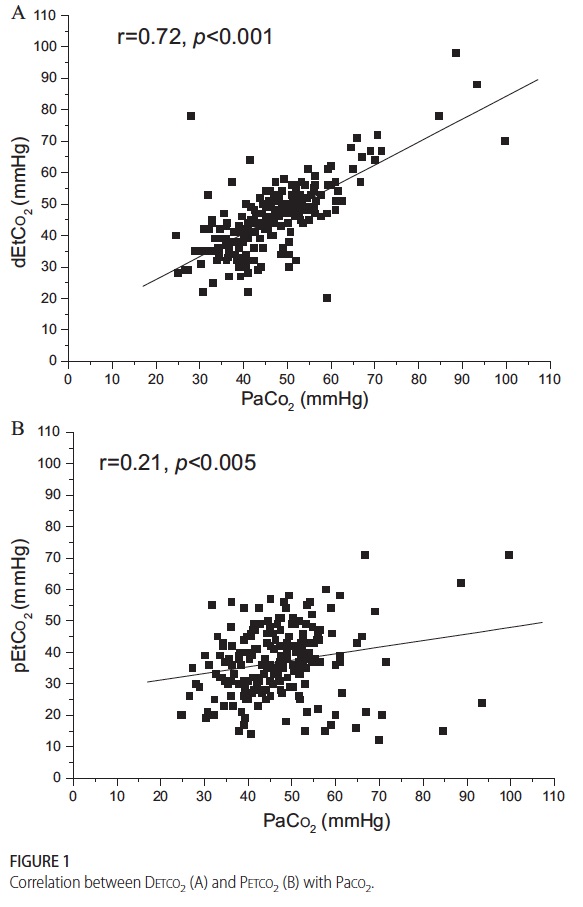 This demonstrates that while proximal measurement was quite poorly correlated with true arterial pCO2 the distal measurement was much more accurate. In fact the mean differences between arterial pCO2 and distal measurement was -1.5 mm Hg while that of proximal measurement -10.2 mm Hg albeit with wide confidence intervals. As found in other studies of proximal end tidal CO2 measurement, worse pulmonary disease correlated with worse accuracy as shown in table 2.
This demonstrates that while proximal measurement was quite poorly correlated with true arterial pCO2 the distal measurement was much more accurate. In fact the mean differences between arterial pCO2 and distal measurement was -1.5 mm Hg while that of proximal measurement -10.2 mm Hg albeit with wide confidence intervals. As found in other studies of proximal end tidal CO2 measurement, worse pulmonary disease correlated with worse accuracy as shown in table 2.
As the pCO2 rises above 60 the accuracy is less but remains much better than proximal measurements. Interestingly the same group has published an additional trial using high frequency ventilation and confirmed the measurements remain accurate. (http://www.ncbi.nlm.nih.gov/pubmed/22328495)
So what does the future hold? in VLBW infants one concern may be the internal diameter of the smallest double lumen tubes and the effect of upsizing to a larger tube and risk of subglottic stenosis. After a personal communication with Dr. Kugelman I understand that this has not been an issue in their unit as they tend to use these double lumen tubes in most if not all of the their infants. The accuracy is sufficient enough from my point of view that units should be able to implement this strategy at least in larger infants at first (those who would need a 3.0 ETT and larger) to see the effect on blood sampling. I suspect that one blood gas a day to determine accuracy in a given patient would be sufficient most of the time if the numbers were found to correlate well.
I would welcome feedback from people who work in units where this strategy has been utilized. How effective is it? Did it reduce your blood gas draws or increase them due to unreliability? Have you seen a rise in subglottic stenosis? Please send your feedback to either this site or at my Facebook page at www.facebook.com/AllThingsNeonatal.