
by All Things Neonatal | Jan 3, 2019 | resuscitation
In July 2016 I published a blog post No more intubating for meconium? Not quite. In this post I highlighted the recent recommendations to modify the approach to the non vigorous infant born through meconium. The traditional approach of electively intubating such infants for tracheal suctioning before beginning PPV was replaced by provision of PPV first. The rationale here was that delaying the establishment of ventilation while trying to intubate for most situations was more risky than just trying to establish a functional residual capacity (FRC). The naysayers pointed out that while this recommendation is possibly warranted for less experienced intubators, perhaps in the hands of those with more skill, tracheal suctioning would be the better option if it could on average be done quickly.
It has been over two years since that recommendation and change in practice. Isn’t it about time someone looked at whether or not this was a good thing to do?
A Comparison of Two Time Periods
Chiruvolu A et al published Delivery Room Management of Meconium-Stained Newborns and Respiratory Support in this month’s Pediatrics. In this paper the authors compared 4 hospitals with a retrospective period of one year before the NRP changes (October 1, 2015, to September 30, 2016) to a one year prospective period (October 1, 2016, to September 30, 2017) after implementation of the new guidelines. In the retrospective cohort there were 11163 mothers delivered at ≥35 weeks’ gestation. Meconium stained amniotic fluid (MSAF) was present in 1303 (12%) deliveries with 130 (10%) of newborns who were nonvigorous. During the prospective time period, a total of 10 717 mothers delivered at ≥35 weeks’ gestation. MSAF was noted in 1282 (12%) deliveries, yielding 101 (8%) newborns who were nonvigorous. Therefore the study compared these 130 newborns in the retrospective cohort to the 101 in the prospective time period. The authors note that aside from the approach to MSAF there were no changes in care during this time in the delivery room.
A few differences exist though in the cohorts that are worth mentioning that were statistically significant. Firstly, the incidence of preterm and post-term infants were both higher in the prospective cohort (both 6% vs 1%). Secondly, the incidence of fetal distress was higher in the prospective cohort 57% vs 43%. All of these factors would tend to favour the retrospective cohort doing better than the prospective and so the authors in their results controlled for these differences. Not surprisingly the rate of intubation in the retrospective group was 70% vs 2% in the prospective arm.
What were the results?
The results shown in table 3 in terms of the Odds ratios have been adjusted for the aforementioned differences of preterm post-term and fetal distress. There are several things here worth noting.  The risk of admission was significantly higher for respiratory distress.
The risk of admission was significantly higher for respiratory distress.
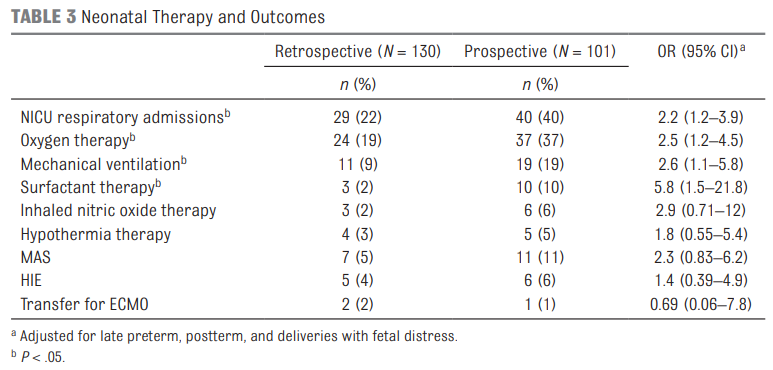 Oxygen needs and mechanical ventilation along with surfactant therapy were also notably higher. One things that showed no difference at all was the mean apgar score at 1 and 5 minutes. This is an interesting finding given the hypothesis that drove the change in practice. If establishing an FRC is the goal of the intervention to provide earlier PPV then shouldn’t the retrospective group have worse apgars due to less effective resuscitation? Maybe or maybe not. This really depends on the staff in the resuscitation room at the 4 hospitals. It might be that the staff were quite skilled so the intubations may have gone smoothly with minimal reductions in FRC compared to the prospective group. What would this study look like if done in a centre with less experienced people capable of intubation.
Oxygen needs and mechanical ventilation along with surfactant therapy were also notably higher. One things that showed no difference at all was the mean apgar score at 1 and 5 minutes. This is an interesting finding given the hypothesis that drove the change in practice. If establishing an FRC is the goal of the intervention to provide earlier PPV then shouldn’t the retrospective group have worse apgars due to less effective resuscitation? Maybe or maybe not. This really depends on the staff in the resuscitation room at the 4 hospitals. It might be that the staff were quite skilled so the intubations may have gone smoothly with minimal reductions in FRC compared to the prospective group. What would this study look like if done in a centre with less experienced people capable of intubation.
Also interesting in this study is that when isolating comparisons to those admitted to the NICU and those specifically diagnosed with MAS there were no differences between groups for such outcomes as length of stay, oxygen therapy, mechanical ventilation (MV) or days of MV. Given that the group sizes though were quite small (7 and 11 for MAS) we do have to take this data with a grain of salt as it really is too small to make any certain conclusions. A larger study would need to be done looking at these types of outcomes to really get a better handle on whether the approach to MSAF matters to these individual outcomes.
Final Thoughts
What this study does for me is raise an eyebrow. The change in practice does not seem to yield “better babies”. Secondly what we do see even when controlling for differences that would affect hospital admissions for respiratory distress is an increase in admission rate. In times when beds are becoming increasingly precious as census for many units swell one has to ask whether this approach is truly the better way to go. Perhaps it was wrong for the NRP to declare that for all practitioners it is best to provide PPV rather than intubate. This may have been too simplistic. If you have experienced intubators perhaps it would be best to continue to intubate first in this setting rather than provide PPV. What this study does is certainly raise questions and begs for a larger study to be done to determine whether these results can be replicated. If they are then I suspect the NRP may be headed down a different path for recommendations yet again.
by All Things Neonatal | Nov 7, 2018 | resuscitation
Much has been written on the topic of cord clamping. There is delayed cord clamping of course but institutions differ on the recommended duration. Thirty seconds, one minute or two or even sometimes three have been advocated for but in the end do we really know what is right? Then there is also the possibility of cord milking which has gained variable traction over the years. A recent review was published here.
Take the Guessing Out of the Picture?
Up until the time of birth there is very little pulmonary blood flow. Typically, about 10% of the cardiac output passes through the lungs and the remained either moves up the ascending aorta or bypasses the lungs via the ductus arteriosus. After birth as the lung expands, pulmonary vascular resistance rapidly decreases allowing cardiac output to take on the familiar pattern which we all live with. Blood returning from the systemic venous circulation no longer bypasses the lung but instead flows through pulmonary capillaries picking up oxygen along the way. One can imagine then that if a baby is born and the cord is clamped right away, blood returning from the systemic circulation continues to bypass the lung which could lead to hypoxemia and reflexive bradycardia. This has been described previously by Blank et al in their paper Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition.
A group of researchers from the Netherlands published a very interesting paper Physiological-based cord clamping in preterm infants using a new purpose-built resuscitation table: a feasibility study this month. The study centres around a resuscitation table called the Concord that is brought to the mother for resuscitation after birth.  The intervention here was applied to infants 26 to 35 weeks gestational age.
The intervention here was applied to infants 26 to 35 weeks gestational age.
The cord was clamped after each of the following was achieved for an infant indicating successful transition with opening of the lung and establishment of an FRC.
1. Establishment of adequate breathing (average tidal volume ≥4 mL/kg) on CPAP. They used a mask capable of measuring expired tidal volumes.
2. HR above 100 bpm
3. SpO2 above 25th percentile using FiO2 <0.4
In this way, the cord was only clamped once the baby appeared to have physiologically made the transition from dependence on umbilical cord blood flow to ventilation perfusion matching in the lung. Although 82 mothers consented only 37 preterm infants were included in the end. Exclusion criteria were signs of placental abruption or placenta praevia, signs of severe fetal distress determined by the clinician and the necessity for an emergency caesarean section ordered to be executed within 15 min. This really was a proof of concept study but the results are definitely worth looking at.
How Did These Babies Do?
There are many interesting findings from this study. The mean time of cord clamping was 4 minutes and 23 seconds (IQR 3:00 – 5:11). Heart rate was 113 (81–143) and 144 (129–155) bpm at 1 min and 5 min
after birth. Only one patient developed bradycardia to <60 BPM but this was during a mask readjustement. The main issue noted as far as adverse events was hypothermia with a mean temperature of 36.0 degrees at NICU admission. Almost 50% of infants had a temperature below 36 degrees. Although the authors clearly indicate that they took measures to prevent heat loss it would appear that this could be improved upon!
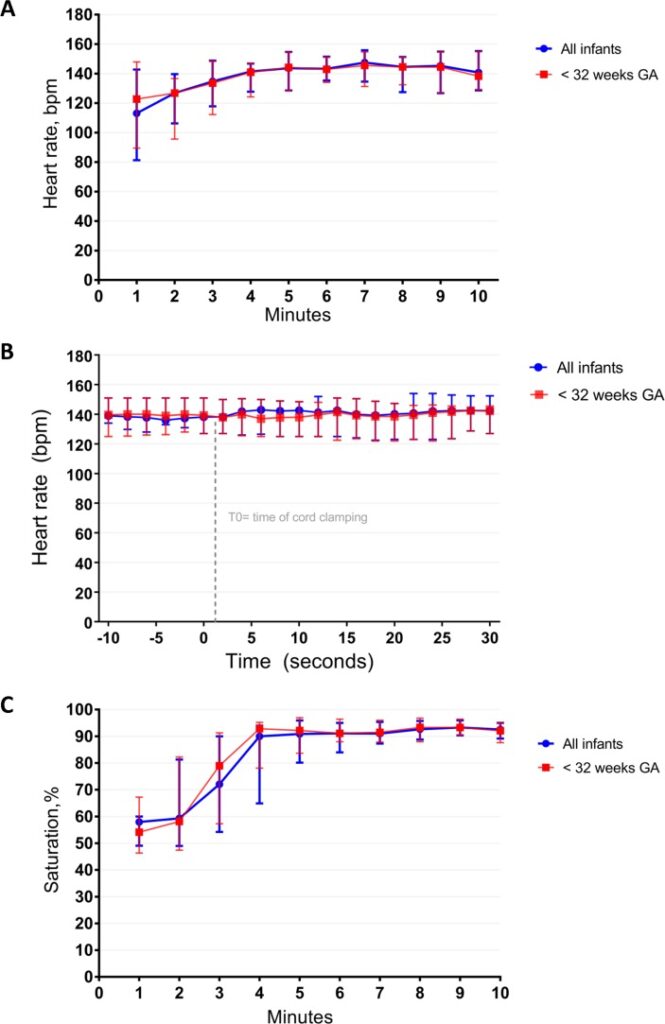
What stands out most to me is the lengthy duration of cord clamping. This study which used a physiologic basis to determine when to clamp a cord has demonstrated that even at 1 minute of waiting that is likely only 1/4 of the time needed to wait for lung expansion to occur to any significant degree. I can’t help but wonder how many of the patients we see between 26-35 weeks who have a low heart rate after delivery might have a higher heart rate if they were given far more time than we currently provide for cord clamping.
I can also see why cord milking may be less effective. Yes, you will increase circulating blood volume which may help with hemodynamic stability but perhaps the key here is lung expansion. You can transfuse all the blood you want but if it has nowhere to go just how effective is it?
As we do more work in this area I have to believe that as a Neonatal community we need to prepare ourselves for the coming of the longer delay for cord clamping. Do we need to really have the “Concord” in every delivery or perhaps it is time to truly look at durations of 3-4 minutes before the team clamps the cord.
Stay tuned!
by All Things Neonatal | Oct 10, 2018 | resuscitation
It has to be one of the most common questions you will hear uttered in the NICU. What were the cord gases? You have a sick infant in front of you and because we are human and like everything to fit into a nicely packaged box we feel a sense of relief when we are told the cord gases are indeed poor. The congruence fits with our expectation and that makes us feel as if we understand how this baby in front of us looks the way they do.
Take the following case though and think about how you feel after reading it. A term infant is born after fetal distress (late deceleration to as low as 50 BPM) is noted on the fetal monitor. The infant is born flat with no heart rate and after five minutes one is detected. By this point the infant has received chest compressions and epinephrine twice via the endotracheal tube. The cord gases are run as the baby is heading off to the NICU for admission and low and behold you get the following results back; pH 7.21, pCO2 61, HCO3 23, lactate 3.5. You find yourself looking at the infant and scratching your head wondering how the baby in front of you that has left you moist with perspiration looks as bad as they do when the tried and true cord gas seems to be betraying you. To make matters worse at one hour of age you get the following result back; pH 6.99, pCO2 55, HCO3 5, lactate 15. Which do you believe? Is there something wrong with the blood gas analyzer?
How Common Is This Situation
You seem to have an asphyxiated infant but the cord gas isn’t following what you expect as shouldn’t it be low due to the fetal distress that was clearly present? It turns out, a normal or mildly abnormal cord gas may be found in asphyxiated infants just as commonly as what you might expect. In 2012 Yeh P et al looked at this issue in their paper The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. The authors sampled a very large number of babies over a near 20 year period to come up with a sample of 51519 babies and sought to pair the results with what they knew of the outcome for each baby.
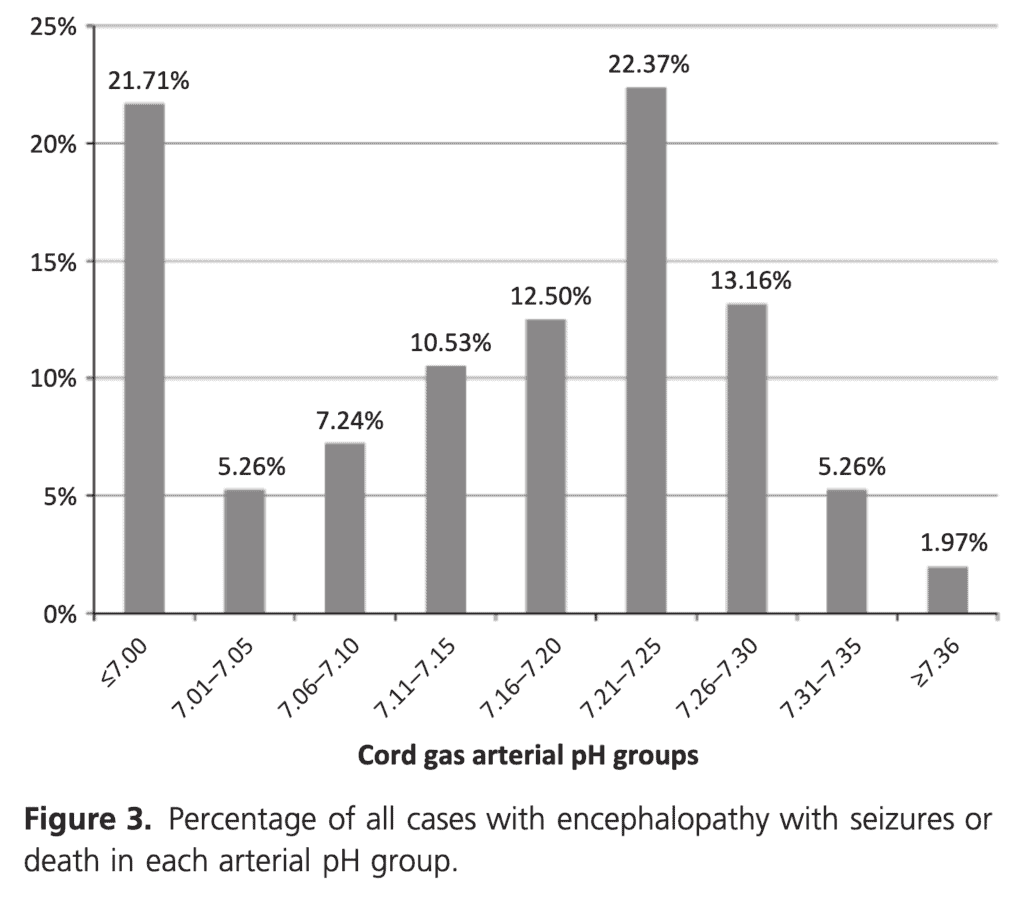
This is where things get interesting. When looking at the outcome of encephalopathy with seizures and/or death you will note that only 21.71% of the babies with this outcome had a gas under 7.00. 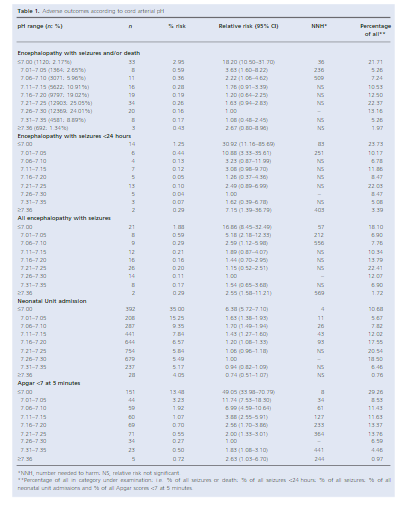
If you include those under 7.10 as still being significantly distressed then this percentage rises to 34.21%. In other words almost 66% of babies who have HIE with seizures and/or death have a arterial cord pH above 7.1! The authors did not look at encephalopathy without seizures but these are the worst infants and almost 2/3 have a cord gas that you wouldn’t much as glance at and say “looks fine”
How do we reconcile this?
The answer lies in the fetal circulation. When an fetus is severely stressed, anaerobic metabolism takes over and produces lactic acid and the metabolic acidosis that we come to expect. For the metabolites to get to the umbilcal artery they must leave the fetal tissues and enter the circulation. If the flow of blood through these tissues is quite poor in the setting of compromised myocardial contractility the acids sit in the tissues. The blood that is therefore sitting in the cord at the time of sampling actually represents blood that was sent to the placenta “when times were good”. When the baby is delivered and we do our job of resuscitating the circulation that is restored then drives the lactic acid into the blood stream and consumes the buffering HCO3 leading to the more typical gases we are accustomed to seeing and reestablishing the congruence our brains so desire. This in fact forms the basis for most HIE protocols which includes a requirement of a cord gas OR arterial blood gas in the first hour of life with a pH < 7.00.
Acidosis May Be Good For the Fetus
To bend your mind just a little further, animal evidence suggests that those fetuses who develop acidosis may benefit from the same and be at an advantage over those infants who don’t get acidemia. Laptook AR et al published Effects of lactic acid infusions and pH on cerebral blood flow and metabolism. In this study of piglets, infusion of lactic acid improved cerebral blood flow. I would suggest improvement in cerebral blood flow of the stressed fetus would be a good thing. Additionally we know that lactate may be used by the fetus as additional metabolic fuel for the brain which under stress would be another benefit. Finally the acidemic fetus is able to offload O2 to the tissues via the Bohr effect. In case you have forgotten this phenomenon, it is the tendency for oxygen to more readily sever its tie to hemoglobin and move into the tissues.
I hope you have found this as interesting as I have in writing it. The next time you see a good cord gas in a depressed infant, pause for a few seconds and ask yourself is this really a good or a bad thing?
by All Things Neonatal | Sep 20, 2018 | resuscitation
One of the first things a student of any discipline caring for newborns is how to calculate the apgar score at birth. Over 60 years ago Virginia Apgar created this score as a means of giving care providers a consistent snapshot of what an infant was like in the first minute then fifth and if needed 10, 15 and so on if resuscitation was ongoing. For sure it has served a useful purpose as an apgar score of 0 and 0 gives one cause for real worry. What about a baby with an apgar of 3 and 7 or 4 and 8? There are certainly infants who have done very well who initially had low apgar scores and conversely those who had higher apgar scores who have had very significant deleterious outcomes including death. I don’t mean to suggest that the apgar scores don’t provide any useful predictive value as they are used as part of the criteria to determine if a baby merits whole body cooling or not. The question is though after 60+ years, has another score been created to provide similar information but enhance the predictive value derived from a score?
The Neonatal Resuscitation and Adaptation Score (NRAS)
Back in 2015 Jurdi et al published Evaluation of a Comprehensive Delivery Room Neonatal Resuscitation and Adaptation Score (NRAS) Compared to the Apgar Score. This new score added into a ten point score resuscitative actions taken at the 1 and 5 minute time points to create a more functional score that included interventions. The other thing this new score addressed was more recent data that indicated a blue baby at birth is normal (which is why we have eliminated asking the question “is the baby pink?” in NRP. Knowing that, the colour of the baby in the apgar score may not really be that relevant. Take for example a baby with an apgar score of 3 at one minute who could have a HR over 100 and be limp, blue and with shallow breathing. Such a baby might get a few positive pressure breaths and then within 10 seconds be breathing quite well and crying. Conversely, they might be getting ongoing PPV for several minutes and need oxygen. Were they also getting chest compressions? If I only told you the apgar score you wouldn’t have much to go on. Now look at the NRAS and compare the information gathered using two cardiovascular (C1&2), one neurological test (N1) and two respiratory assessments (R1&2).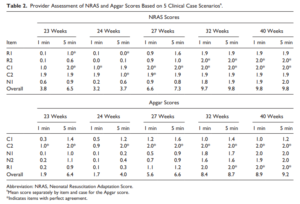

The authors in this study performed a pilot study on only on 17 patients really as a proof of concept that the score could be taught and implemented. Providers reported both scores and found “superior interrater reliability (P < .001) and respiratory component reliability (P < .001) for all gestational ages compared to the Apgar score.”
A Bigger Study Was Needed
The same group in 2018 this time led by Witcher published Neonatal Resuscitation and Adaptation Score vs Apgar: newborn assessment and predictive ability. The primary outcome was the ability of a low score to predict mortality with a study design that was a non-inferiority trial. All attended deliveries were meant to have both scores done but due to limited numbers of trained personnel who could appropriately administer both scores just under 90% of the total deliveries were assigned scores for comparison. The authors sought to recruit 450 infants to show that a low NRAS score (0–3) would not be inferior to a similar Apgar at predicting death. Interestingly an interim analysis found the NRAS to be superior to Apgar when 75.5% of the 450 were enrolled, so the study was stopped. What led the apgar score to perform poorly in predicting mortality (there were only 12 deaths though in the cohort) was the fact that 49 patients with a 1 minute apgar score of 0-3 survived compared to only 7 infants with a low NRAS score.
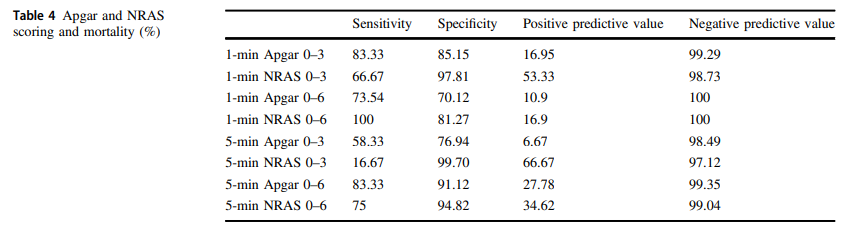
The other interesting finding was the ability of the NRAS to predict the need for respiratory support at 48 hours with a one minute apgar score of 0-3 being found in 39% of those on support compared to 100% of those with a low NRAS. Also at 5 minutes a score of 4-6 for the apgar was found in 48% of those with respiratory support at 48 hours vs 87% of those with a similar range NRAS. These findings were statistically significant while a host of other conditions such as sepsis, hypoglycemia, hypothermia and others were no different in terms of predictive ability of the scores.
An Even Bigger Study is Needed
To be sure, this study is still small and missed just over 90% of all deliveries so it is possible there is some bias that is not being detected here. I do think there is something here though which a bigger study that has an army of people equipped to provide the scoring will add to this ongoing story. Every practitioner who resuscitates an infant is asked at some point in those first minutes to hour “will my baby be ok?”. The truth is that the apgar score has never lived up to the hope that it would help us provide an accurate clairvoyant picture of what lies ahead for an infant. Where this score gives me hope is that a score which would at the very least help me predict whether an infant would likely still be needing respiratory support in 48 hours provides the basic answer to the most common question we get in the unit once admitted; “when can I take my baby home”. Using this score I could respond with some greater confidence in saying “I think your infant will be on support for at least 48 hours”. The bigger question though which thankfully we don’t have to address too often for the sickest babies at birth is “will my baby survive?”. If a larger study demonstrates this score to provide a greater degree of accuracy then the “Tipping Point” might just be that to switching over to the NRAS and leaving the apgar score behind. That will never happen overnight but medicine is always evolving and with time you the reader may find yourself becoming very familiar with this score!
by All Things Neonatal | Jul 26, 2018 | Neonatal, Neonatology, resuscitation
It is hard to believe but it has been almost 3 years since I wrote a piece entitled A 200 year old invention that remains king of all tech in newborn resuscitation. In the post I shared a recent story of a situation in which the EKG leads told a different story that what our ears and fingers would want us to believe. The concept of the piece was that in the setting of pulseless electrical activity (where there is electrical conductance in the myocardium but lack of contraction leaves no blood flow to the body) one could pick up a signal from the EKG leads when there is in fact no pulse or perfusion to vital organs. This single experience led me to postulate that this situation may be more common than we think and the application of EKG leads routinely could lead to errors in decision making during resuscitation of the newborn. It is easy to see how that could occur when you think about the racing pulses of our own in such situations and once chest compressions start one might watch the monitor and forget when they see a heart rate of 70 BPM to check for a corresponding pulse or listen with the stethoscope. I could see for example someone stopping chest compressions and continuing to provide BVM ventilation despite no palpable pulse when they see the QRS complex clearly on the monitor. I didn’t really have much evidence to support this concern but perhaps there is a little more to present now.
A Crafty Animal Study Provides The Evidence
I haven’t presented many animal studies but this one is fairly simple and serves to illustrate the concern in a research model. For those of you who haven’t done animal research, my apologies in advance as you read what happened to this group of piglets. Although it may sound awful, the study has demonstrated that the concern I and others have has is real.
For this study 54 newborn piglets (equivalent to 36-38 weeks GA in humans) were anesthetized and had a flow sensor surgically placed around the carotid artery. ECG leads were placed as well and then after achieving stabilization, hypoxia was induced with an FiO2 of 0.1 and then asphyxia by disconnecting the ventilator and clamping the ETT. By having a flow probe around the carotid artery the researchers were able to determine the point of no cardiac output and simultaneously monitor for electrical activity via the EKG leads. Auscultation for heart sounds was performed as well.
The results essentially confirm why I have been concerned with an over reliance on EKG leads.

Of the 57 piglets, 14 had asystole and no carotid flow but in 23 there was still a heart rate present on the EKG with no detectable carotid flow. This yields a sensitivity of only 37%. Moreover, the overall accuracy of the ECG was only 56%.
Meanwhile the stethoscope which I have referred to previously as the “king” in these situations had 100% sensitivity so remains deserving of that title.
What do we do with such information?
I think the results give us reason to pause and remember that faster isn’t always better. Previous research has shown that signal acquisition with EKG leads is faster than with oximetry. While a low heart rate detected quickly is helpful to know what the state of the infant is and begin the NRP pathway, we simply can’t rely on the EKG to tell us the whole story. We work in interdisciplinary teams and need to support one another in resuscitations and provide the team with the necessary information to perform well. The next time you are in such a situation remember that the EKG is only one part of the story and that auscultation for heart sounds and palpation of the umbilical cord for pulsation are necessary steps to demonstrate conclusively that you don’t just have a rhythm but a perfusing one.
I would like to thank the Edmonton group for continuing to put out such important work in the field of resuscitation!
by All Things Neonatal | May 30, 2018 | intubation, Neonatal, Neonatology, preemie, Prematurity, resuscitation
If I look back on my career there have been many things I have been passionate about but the one that sticks out as the most longstanding is premedicating newborns prior to non-emergent intubation. The bolded words in the last sentence are meant to reinforce that in the setting of a newborn who is deteriorating rapidly it would be inappropriate to wait for medications to be drawn up if the infant is already experiencing severe oxygen desaturation and/or bradycardia. The CPS Fetus and Newborn committee of which I am a member has a statement on the use of premedication which seems as relevant today as when it was first developed. In this statement the suggested cocktail of atropine, fentanyl and succinylcholine is recommended and having used it in our centre I can confirm that it is effective. In spite of this recommendation by our national organization there remain those who are skeptical of the need for this altogether and then there are others who continue to search for a better cocktail. Since I am at the annual conference for the CPS in Quebec city  I thought it would be appropriate to provide a few comments on this topic.
I thought it would be appropriate to provide a few comments on this topic.
Three concerns with rapid sequence induction (RSI) for premedication before intubation
1. “I don’t need it. I don’t have any trouble intubating a newborn” – This is perhaps the most common reason I hear naysayers raise. There is no question that an 60-90 kg practitioner can overpower a < 5kg infant and in particular an ELBW infant weighing < 1 kg. This misses the point though. Premedicating has been shown to increase success on the first attempt and shorten times to intubation. Dempsey 2006, Roberts 2006, Carbajal 2007, Lemyre 2009
2. “I usually get in on the first attempt and am very slick so risk of injury is less.” Not really true overall. No doubt there are those individuals who are highly successful but overall the risk of adverse events is reduced with premedication. (Marshall 1984, Lemyre 2009). I would also proudly add another Canadian study from Edmonton by Dr. Byrne and Dr. Barrington who performed 249 consecutive intubations with predication and noted minimal side effects but high success rates at first pass.
3. “Intubation is not a painful procedure”. This one is somewhat tough to obtain a true answer for as the neonate of course cannot speak to this. There is evidence available again from Canadian colleagues in 1984 and 1989 that would suggest that infants at the very least experience discomfort or show physiologic signs of stress when intubated using an “awake” approach. In 1984 Kelly and Finer in Edmonton published Nasotracheal intubation in the neonate: physiologic responses and effects of atropine and pancuronium. This randomized study of atropine with or without pancuronium vs control demonstrated intracranial hypertension only in those infants in the control arm with premedication ameliorating this finding. Similarly, in 1989 Barrington, Finer and the late Phil Etches also in Edmonton published Succinylcholine and atropine for premedication of the newborn infant before nasotracheal intubation: a randomized, controlled trial. This small study of 20 infants demonstrated the same finding of elimination of intracranial hypertension with premedication. At the very least I would suggest that having a laryngoscope blade put in your oral cavity while awake must be uncomfortable. If you still doubt that statement ask yourself whether you would want sedation if you needed to be intubated? Still feel the same way about babies not needing any?
4. What if I sedate and paralyze and there is a critical airway? Well this one may be something to consider. If one knows there is a large mass such as a cystic hygroma it may be best to leave the sedation or at least the paralysis out. The concern though that there might be an internal mass or obstruction that we just don’t know about seems a little unfounded as a justification for avoiding medications though.
Do we have the right cocktail?
The short answer is “I don’t know”. What I do know is that the use of atropine, an opioid and a muscle relaxant seems to provide good conditions for intubating newborns. We are in the era of refinement though and as a recent paper suggests, there could be alternatives to consider;Effect of Atropine With Propofol vs Atropine With Atracurium and Sufentanil on Oxygen Desaturation in Neonates Requiring Nonemergency IntubationA Randomized Clinical Trial. I personally like the idea of a two drug combination for intubating vs.. three as it leaves one less drug to worry about a medication error with. There are many papers out there looking at different drug combinations. This one though didn’t find a difference between the two combinations in terms of prolonged desaturations between the two groups which was the primary outcome. Interestingly though the process of intubating was longer with atropine and propofol. Given some peoples reluctance to use RSI at all, any drug combination which adds time to the the procedure is unlikely to go over well. Stay tuned though as I am sure there will be many other combinations over the next few years to try out!

 The risk of admission was significantly higher for respiratory distress.
The risk of admission was significantly higher for respiratory distress. Oxygen needs and mechanical ventilation along with surfactant therapy were also notably higher. One things that showed no difference at all was the mean apgar score at 1 and 5 minutes. This is an interesting finding given the hypothesis that drove the change in practice. If establishing an FRC is the goal of the intervention to provide earlier PPV then shouldn’t the retrospective group have worse apgars due to less effective resuscitation? Maybe or maybe not. This really depends on the staff in the resuscitation room at the 4 hospitals. It might be that the staff were quite skilled so the intubations may have gone smoothly with minimal reductions in FRC compared to the prospective group. What would this study look like if done in a centre with less experienced people capable of intubation.
Oxygen needs and mechanical ventilation along with surfactant therapy were also notably higher. One things that showed no difference at all was the mean apgar score at 1 and 5 minutes. This is an interesting finding given the hypothesis that drove the change in practice. If establishing an FRC is the goal of the intervention to provide earlier PPV then shouldn’t the retrospective group have worse apgars due to less effective resuscitation? Maybe or maybe not. This really depends on the staff in the resuscitation room at the 4 hospitals. It might be that the staff were quite skilled so the intubations may have gone smoothly with minimal reductions in FRC compared to the prospective group. What would this study look like if done in a centre with less experienced people capable of intubation.

 The intervention here was applied to infants 26 to 35 weeks gestational age.
The intervention here was applied to infants 26 to 35 weeks gestational age.










 I thought it would be appropriate to provide a few comments on this topic.
I thought it would be appropriate to provide a few comments on this topic.