
by All Things Neonatal | May 9, 2018 | hypoglycemia, Neonatal, Neonatology
 I have written a number of times already on the topic of dextrose gels. Previous posts have largely focused on the positive impacts of reduction in NICU admissions, better breastfeeding rates and comparable outcomes for development into childhood when these gels are used. The papers thus far have looked at the effectiveness of gel in patients who have become hypoglycemic and are in need of treatment. The question then remains as to whether it would be possible to provide dextrose gel to infants who are deemed to be at risk of hypoglycemia to see if we could reduce the number of patients who ultimately do become so and require admission.
I have written a number of times already on the topic of dextrose gels. Previous posts have largely focused on the positive impacts of reduction in NICU admissions, better breastfeeding rates and comparable outcomes for development into childhood when these gels are used. The papers thus far have looked at the effectiveness of gel in patients who have become hypoglycemic and are in need of treatment. The question then remains as to whether it would be possible to provide dextrose gel to infants who are deemed to be at risk of hypoglycemia to see if we could reduce the number of patients who ultimately do become so and require admission.
Answering that question
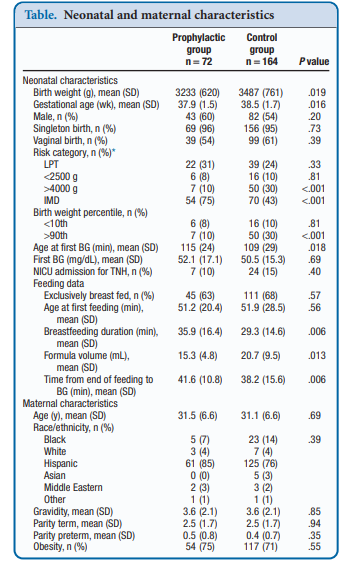
Recently, Coors et al published Prophylactic Dextrose Gel Does Not Prevent Neonatal Hypoglycemia: A Quasi-Experimental Pilot Study. What they mean by Quasi-Experimental is that due to availability of researchers at off hours to obtain consent they were unable to produce a randomized controlled trial. What they were able to do was compare a group that had the following risk factors (late preterm, birth weight <2500 or >4000 g, and infants of mothers with diabetes) that they obtained consent for giving dextrose gel following a feed to a control group that had the same risk factors but no consent for participation. The protocol was that each infant would be offered a breastfeed or formula feed after birth followed by 40% dextrose gel (instaglucose) and then get a POC glucose measurement 30 minutes later. A protocol was then used based on different glucose results to determine whether the next step would be a repeat attempt with feeding and gel or if an IV was needed to resolve the issue.
To be sure, there was big hope in this study as imagine if you could prevent a patient from becoming hypoglycemic and requiring IV dextrose followed by admission to a unit. Sadly though what they found was absolutely no impact of such a strategy. Compared with the control group there was no difference in capillary glucose after provision of dextrose gel (52.1 ± 17.1 vs 50.5 ± 15.3 mg/dL, P = .69).  One might speculate that this is because there are differing driving forces for hypoglycemia and indeed that was the case here where there were more IDMs and earlier GA in the prophylactic group. On the other hand there were more LGA infants in the control group which might put them at higher risk. When these factors were analyzed though to determine whether they played a role in the lack of results they were found not to. Moreover, looking at rates of admission to the NICU for hypoglycemia there were also no benefits shown. Some benefits were seen in breastfeeding duration and a reduction in formula volumes consistent with previous studies examining the effect of glucose gel on both which is a win I suppose.
One might speculate that this is because there are differing driving forces for hypoglycemia and indeed that was the case here where there were more IDMs and earlier GA in the prophylactic group. On the other hand there were more LGA infants in the control group which might put them at higher risk. When these factors were analyzed though to determine whether they played a role in the lack of results they were found not to. Moreover, looking at rates of admission to the NICU for hypoglycemia there were also no benefits shown. Some benefits were seen in breastfeeding duration and a reduction in formula volumes consistent with previous studies examining the effect of glucose gel on both which is a win I suppose.
It may also be that when you take a large group of babies with risks for hypoglycemia but many were never going to become hypoglycemic, those who would have had a normal sugar anyway dilute out any effect. These infants have a retained ability to produce insulin in response to a rising blood glucose and to limit the upward movement of their glucose levels. As such what if the following example is at work? Let’s say there are 200 babies who have risk factors for hypoglycemia and half get glucose gel. Of the 100 about 20% will actually go on to have a low blood sugar after birth. What if there is a 50% reduction in this group of low blood sugars so that only 10 develop low blood glucose instead of 20. When you look at the results you would find in the prophylaxis group 10/100 babies have a low blood sugar vs 20/100. This might not be enough of a sample size to demonstrate a difference as the babies who were destined not to have hypoglycemia dilute out the effect. A crude example for sure but when the incidence of the problem is low, such effects may be lost.
A Tale of Two Papers
This post is actually part of a series with this being part 1. Part 2 will look at a study that came up with a different conclusion. How can two papers asking the same question come up with different answers? That is the story of medicine but in the next part we will look at a paper that suggests this strategy does work and look at possible reasons why.

by All Things Neonatal | Apr 4, 2018 | hypoglycemia, Neonatal, Neonatology
Hypoglycemia has to be one of the most common conditions that we screen for or treat in the NICU and moreover in newborn care in general. The Canadian Pediatric Society identifies small for gestational age infants (weight <10th percentile), large for gestational age (LGA; weight > 90th percentile) infants, infants of diabetic mothers (IDMs) and preterm infants as being high risk for hypoglycemia. It is advised then to screen such babies in the absence of symptoms for hypoglycemia 2 hours after birth after a feed has been provided (whether by breast or bottle). I am sure though if you ask just about any practitioner out there, they will tell you a story about a baby with “no risk factors” who had hypoglycemia. These one-off cases have the effect though of making us want to test everyone for fear that we will miss one. If that is the case though should we be recommending that all babies get at least one check?
The Canadian Pediatric Surveillance Program (CPSP)
The CPSP is a branch of the Canadian Pediatric Society that “provides an innovative means to undertake active paediatric surveillance and increase awareness of childhood disorders that are high in disability, morbidity, mortality and economic cost to society, despite their low frequency. I submit my surveys each month as i hope other Canadian Pediatricians do and help to determine the impact of these rare conditions in our Canadian population. Like with any survey we rely on people taking the time to submit but there is always the risk that what is being sent in under represents the true burden of illness as some cases may not be identified. Having said that, it is the best we have!
Turning our attention to hypoglycemia in low risk newborns
From April 2014 to March 2016 the CPSP searched for these types of patients and just published the results of their findings in Hypoglycemia in unmonitored full-term newborns—a
surveillance study by Flavin MP et al. What I like about the study is that they have been able to look at a group of babies that fall outside those identified as being at risk in the CPS statement Screening guidelines for newborns at risk for low blood glucose. They were looking for severe hypoglycemia by using a threshold of < 2.0 mmol/L (36 mg/dl) and all infants must have received IV dextrose. In the end after excluding ineligible cases they had 93 babies who met criteria. Based on the Canadian birth rate this translates to an incidence of 1 in every 8378 births. These babies were all supposed to be low risk but there were in fact clues that while not strictly identified as risks in the CPS statement could have increased the likelihood of a low blood glucose. Twenty three percent of mothers had maternal hypertension and another 23% were obese while 47% had excessive weight gain during pregnancy. Furthermore, 8% of mothers were treated with a beta blocker (most likely labetalol I would think) during pregnancy which is a risk factor for hypoglycemia although not specifically cited in the current CPS statement.
A concerning finding as well was the likelihood of severe symptoms in this group on presentation. Twenty percent presented with major clinical signs (seizure, apnea or cyanosis). Median glucose levels at presentation were much lower than those without major signs (median = 0.8 mmol/L, interquartile range [IQR] = 0.5 versus 1.6 mmol/L, IQR = 0.7; P < 0.001). Lastly, providers were asked about neurodevelopmental concerns at discharge approximately 20% were thought to have issues.
Are these patients really low risk though?
Twenty five percent of the patients submitted had a birth weight less than the 10%ile for GA. These patients as per the CPS guideline recommendations are actually considered at risk and should have been screened. The second issue to address has to do with the way we diagnose diabetes in pregnancy. All women are provided with the oral glucose tolerance test around 28 weeks of pregnancy. No test is perfect but it is the best we have. Women who have excessive weight gain in pregnancy (almost 50% of the cohort) are at higher risk of developing diabetes or some degree of insulin resistance as are those who are classified as obese. I have long suspected and think it may be the case here that some babies who do not meet the criteria for screening as their mothers do not have a diagnosis of GDM actually are at risk due to some degree of insulin resistance or perhaps their mothers develop GDM later. The evidence for this are the occasional LGA babies who are born to mothers without a GDM diagnosis but who clearly have been exposed to high insulin levels as they behave like such affected infants with poor feeding and low sugars in the newborn period. The authors here comment on those that were SGA but how many in this cohort were LGA?
The effect of hypertension can also not be minimized which was present in about a quarter of patients. These babies while not being officially SGA may have experienced a deceleration in weight gain in the last few weeks but remained above the 10%ile. These infants would not have the glycogen stores to transition successfully but would not be targeted as being at risk by the current definitions.
Should we be screening everyone then?
If we acknowledge that about 25% were IUGR in this study (<10%ile) and should have been screened, the expected rate would be 1:1170 births alone. In Manitoba with our 17000 births a year we would capture about two extra babies a year which translates into a low of pokes for a lot of healthy babies. Given the further information that 1:5 babies who are identified may have neurodevelopmental concerns it would take about 2-3 years of testing to prevent one concern. That pick up rate for me is far too low to subject so many babies to testing. What this study though does highlight is the need to view risk factors a little less strictly. Babies who are almost meeting the criteria for being LGA or those whose mother’s have taken lebetalol should have a low threshold for screening. Should hypertension on medications, excessive maternal weight gain or obesity in the mother be considered a risk? What I didn’t see in the end of this study were patients who truly were AGA, being born to healthy non overweight mothers presenting as high risk.
Maybe what is really needed based on this study is to re-evaluate what we consider at risk. In the meantime, maybe we should be testing a few extra babies who fall into these “lesser” risk categories. Better yet a study isolating such patients and looking at the frequency of hypoglycemia in these patients is warranted to get a better idea of whether they are indeed risks.

by All Things Neonatal | Feb 8, 2018 | hypoglycemia
Hypoglycemia has to be one of the most common conditions that we treat in the newborn admitted to NICU. For many infants the transitional phase of hypoglycemia can be longer than a couple low blood sugars and as nurses commonly express, it doesn’t take long before the heels of these infants begin to resemble hamburger. For those of you who have used diazoxide in the treatment of hypoglycemia you know that it works and it works quickly to raise the blood sugar. It works by blocking the production of insulin from the pancreas, so particularly in the setting of an infant with detectable insulin levels while hypoglycemic (should be undetectable with a low blood sugar) it can be quite effective. In my own practice I have found that often within one or two doses of the medication with treatment being 5-15 mg/kg/d it can seem to work miracles. Years ago I heard rumours of a trial from birth of this medication in infants of diabetic mothers but saw nothing come to fruition. As someone though who really strives to critically look at every needle poke and strongly consider the need I have always leaned towards the use of this medication if only to reduce what I suspected would be a large number of heel lances.
A Study Comes Forward
Balachandran B et al published a paper on this topic this week in Acta Paediatrica entitled Randomised controlled trial of diazoxide for small for gestational age neonates with hyperinsulinaemic hypoglycaemia provided early hypoglycaemic control without adverse effects. To be clear this is a very small study with only 30 patients in total (15 in the diazoxide and 15 in the placebo arms) and as they had nothing to go on for determining a sample size needed there was no power calculation. The authors chose to look at a very specific group of neonates that were SGA and had hyperinsulinemic hypoglycemia so we need to resist extrapolating to other patient groups such as IDMs in case there is a positive effect here.
With those warnings though, what they did was devise a stepwise approach to initiating diazoxide at 8 mg/kg/d and escalating the dose to as much as 12 mg/kg/d followed by a standardized wean following blood glucose stability. The primary outcome in this case was the number of hours required to achieve a stable glucose with a glucose infusion rate of =< 4mg/kg/min. They examined a number of secondary outcomes as well including duration of IV fluids, episodes of sepsis and time to achieve full feeds as well as mortality. Given the small sample size though I would resist drawing too many conclusions about these secondary outcomes but they are reported nonetheless. 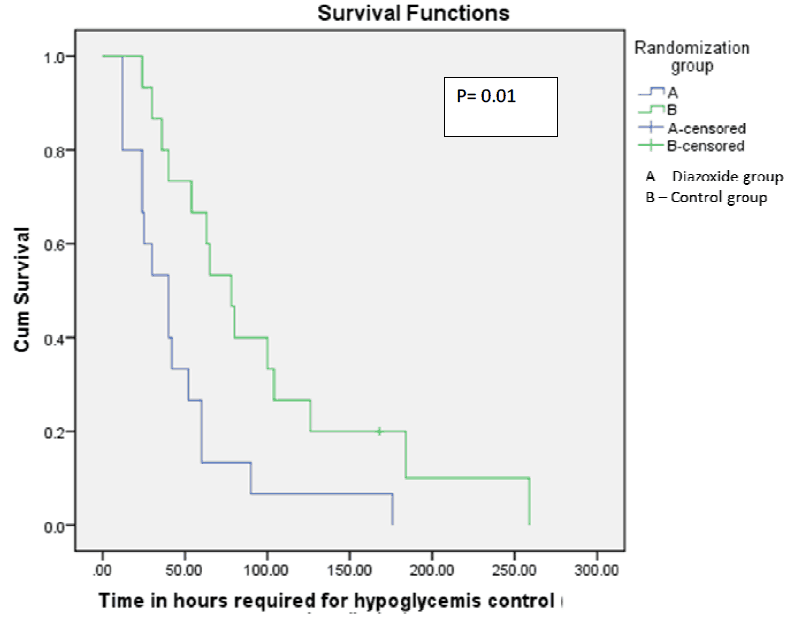 From the paper the Kaplan Meier curve indicates a faster time to stability of blood sugars for 6 hours favouring the diazoxide group. Importantly there were no differences in baseline insulin or cortisol levels between the groups which might explain differing times to glycemic control. Intravenous reductions with feeding increments were also standardized for the study to ensure comparable treatment strategies aside from the provided diazoxide or placebo.
From the paper the Kaplan Meier curve indicates a faster time to stability of blood sugars for 6 hours favouring the diazoxide group. Importantly there were no differences in baseline insulin or cortisol levels between the groups which might explain differing times to glycemic control. Intravenous reductions with feeding increments were also standardized for the study to ensure comparable treatment strategies aside from the provided diazoxide or placebo.
Claim of Safety
The authors note there were no differences in mortality or number of sepsis episodes between the groups. They did find a statistically significant reduction in duration of IV fluid requirements which is likely believable despite my earlier warning as the length of time to achieve control was significantly reduced. The fact remains though with such few patients I would take claims of safety with a grain of salt. You might think at this point though that I would be a champion for the therapy but despite my earlier enthusiasm I do have some reservations. The median time to achieve glycemic control was 40 vs 72.5 hours with a p value of 0.015 which is certainly significant but really we are talking about nearly 2 vs 3 days of management. Is diazoxide truly safe enough to warrant the 30 hour reduction in time to glycemic control? Assuming q3h point of care glucose checks this would be about 8-10 less pokes as a best case scenario but more likely 4-6 less as near the end of checking glucoses as the patient becomes more stable the number of pokes usually decreases. Is diazoxide worth it though?

Back in 2015 the FDA issued a warning that diazoxide can lead to pulmonary hypertension. In truth we have seen it in babies where I practice and as such now routinely have an ECHO done before starting the drug to determine if there is any pulmonary hypertension prior to starting the drug and if there is even a hint it is contraindicated. It isn’t too common a complication as in the FDA bulletin (read here) there have been only 11 cases reported since 1973 but it is a risk nonetheless.
Thirty patients sadly isn’t enough to rule out this complication and it is worth nothing that the authors did not look for this outcome so we don’t know if any patients suffered this.
Am I saying that one should never use diazoxide? Absolutely not but I am suggesting that if you use it then use it with great caution. Although I am delighted the authors chose to perform this study taking all risks into account and looking at the benefit in terms of time on IV and that needed to gain control of blood sugars I can’t say this should be standard of care.
by All Things Neonatal | Nov 23, 2017 | hypoglycemia
We sure do poke a lot of babies to test their blood glucose levels. Some of these babies don’t have so much blood to spare either so checking sugars multiple times a day can drain the body of that precious blood they so need for other functions. Taking too much can always be addressed with a blood transfusion but that as I see it may be avoidable so shouldn’t we do what we can to cut down on blood work? Those with diabetes will be familiar with a continuous glucose monitor (CGM) which is implanted in the skin and can stay in place up to 7 days. The device does require calibration twice a day with a capillary sample to verify it is reading well but this saves a couple pokes a day for those who check four times a day. Such a device could be useful in the NICU where those with hypoglycemia may be checked 6 or more times per day if they are either hypo or hyperglycemic. Cutting this down to two a day would certainly we something worth striving for and if not for the reduction in blood loss then for the minimization of painful procedures.
Does it work in small babies?
A natural question for sure.Uettweller et al published Real-time continuous glucose monitoring reduces the duration of hypoglycemia episodes: a randomized trial in very low birth weight neonates. In babies with a BW < 1500g they were able to demonstrate in 43 babies (21 with traditional intermittent glucose checks vs 22 with CGM) a reduction in duration of hypoglycemia episodes per patient (CGM 44[10-140] min versus IGM 95[15-520] min, p<0.05). Moreover in this brief study of the first three days of life they also found a reduction in the total number of pokes per patient of 5 pokes (22 vs 16). The numbers however are small and the duration short in only being three days so it did not provide a perfect answer as to whether this technology would work in babies from 500-750g reliably but certainly for older babies, continuous knowledge of the blood glucose in theory would allow for faster response to low sugars and as a result as evidenced by the results led to a decrease in time with a low blood glucose.
Improving on these results
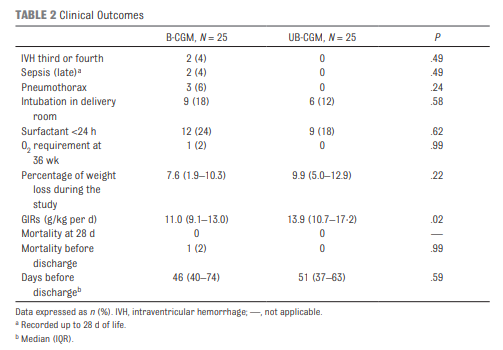
Galderisi et al just published Continuous Glucose Monitoring in Very Preterm Infants: A Randomized Controlled Trial. The study remains small at 50 and the target group ranging from 28-31 weeks (all < 1500g) but the study followed babies for a longer time frame of 7 days. This study employed an algorithm for adjustments in glucose infusion that required staff to first put data into an excel spreadsheet and then the predictive algorithm dictated whether to increase or decrease the rate of dextrose infusion. In one arm, CGM results were unblinded and the practitioners relied on the rate of change to determine the predicted glucose 15 minutes into the future while in the blinded group the CGM was used but results were not available (retrospectively yes) so changes were made based on the usual practice of obtaining point of care results and modifying glucose infusion rates based on that result. The primary outcome of interest here was percentage of time in the euglycemic range of 72 – 144 mg/dL (4-8 mmol/L). Secondary outcomes were time spent hypo or hyperglycemic (mild hypoglycemia (M-HYPO) (47–71 mg/dL); severe hypoglycemia (S-HYPO) (<47 mg/dL); mild hyperglycemia (M-HYPER) (145–180 mg/dL); and severe hyperglycemia (S-HYPER) (>180 mg/dL)). The study lasted a total of seven days allowing the use of one subcutaneous probe per patient as they can last one week after insertion.
How did the approaches compare?
As you might have expected, having a predictive model proved superior. Overall after adjusting for sex, gestational age and weight mean time in target using the unblinded CGM was 83% [95% CI, 79%–87%] and of 71% [95% CI, 67%–76%] in B-CGM [P < .001]). 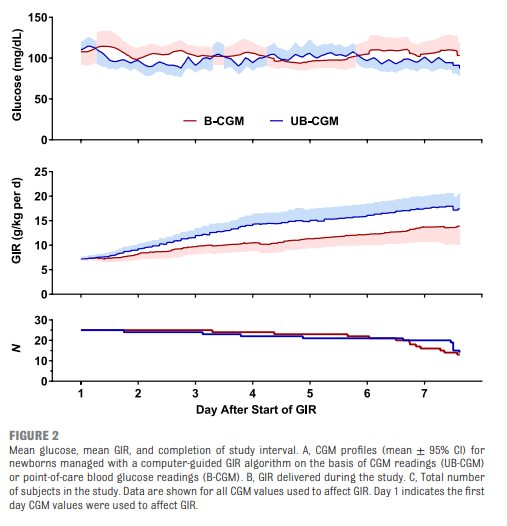

As for secondary outcomes one can see that the time spent in the hypo/hyper areas was much less as a percentage of time than using traditional methods of intermittent sampling. One interesting outcome was that the total number of samples used over the study was an average of 2.4 tests per day in the unblinded group vs 2.59 per day in the intermittent sampling group which although statistically different does not seem to have much clinical impact.
A few questions remain
The idea of using an implanted CGM for infants in the NICU is one that excites me. The lack of a reduction in pokes in a meaningful way is disappointing but I can’t help but wonder if the effect was different whether you were in the first or second half of the week. What if glycemic control in these 29-31 week infants had stabilized by 2-3 days such that you only needed one or two glucose checks in the last half of the week per day? The CGM requires calibration twice daily with POC samples so the lack of a difference my be due to those issues. Future, calibration is rumoured to be possible with one sample so that may change.
There is no disputing though that the use of the predictive algorithm made a difference in terms of avoidance of hypo/hyperglycemic episodes. A larger study would be needed to look at whether this impacts harm that may be associated with such variability such as IVH or ROP but it certainly is promising. The biggest issue here is that I cannot see people manually inputting glucose readings on the CGM into an excel sheet in everyday practice. For this to become widely adopted, a simplified approach to prediction would be required or even better a feedback loop whereby data from the CGM would relay to the infusion pump and rates adjusted automatically (with manual override available).
The use of CGM is coming though and I can’t help but think in the larger babies born to mothers with diabetes there would be a real heal sparing effect with these. Might this be the next study?

 I have written a number of times already on the topic of dextrose gels. Previous posts have largely focused on the positive impacts of reduction in NICU admissions, better breastfeeding rates and comparable outcomes for development into childhood when these gels are used. The papers thus far have looked at the effectiveness of gel in patients who have become hypoglycemic and are in need of treatment. The question then remains as to whether it would be possible to provide dextrose gel to infants who are deemed to be at risk of hypoglycemia to see if we could reduce the number of patients who ultimately do become so and require admission.
I have written a number of times already on the topic of dextrose gels. Previous posts have largely focused on the positive impacts of reduction in NICU admissions, better breastfeeding rates and comparable outcomes for development into childhood when these gels are used. The papers thus far have looked at the effectiveness of gel in patients who have become hypoglycemic and are in need of treatment. The question then remains as to whether it would be possible to provide dextrose gel to infants who are deemed to be at risk of hypoglycemia to see if we could reduce the number of patients who ultimately do become so and require admission. One might speculate that this is because there are differing driving forces for hypoglycemia and indeed that was the case here where there were more IDMs and earlier GA in the prophylactic group. On the other hand there were more LGA infants in the control group which might put them at higher risk. When these factors were analyzed though to determine whether they played a role in the lack of results they were found not to. Moreover, looking at rates of admission to the NICU for hypoglycemia there were also no benefits shown. Some benefits were seen in breastfeeding duration and a reduction in formula volumes consistent with previous studies examining the effect of glucose gel on both which is a win I suppose.
One might speculate that this is because there are differing driving forces for hypoglycemia and indeed that was the case here where there were more IDMs and earlier GA in the prophylactic group. On the other hand there were more LGA infants in the control group which might put them at higher risk. When these factors were analyzed though to determine whether they played a role in the lack of results they were found not to. Moreover, looking at rates of admission to the NICU for hypoglycemia there were also no benefits shown. Some benefits were seen in breastfeeding duration and a reduction in formula volumes consistent with previous studies examining the effect of glucose gel on both which is a win I suppose.


 From the paper the Kaplan Meier curve indicates a faster time to stability of blood sugars for 6 hours favouring the diazoxide group. Importantly there were no differences in baseline insulin or cortisol levels between the groups which might explain differing times to glycemic control. Intravenous reductions with feeding increments were also standardized for the study to ensure comparable treatment strategies aside from the provided diazoxide or placebo.
From the paper the Kaplan Meier curve indicates a faster time to stability of blood sugars for 6 hours favouring the diazoxide group. Importantly there were no differences in baseline insulin or cortisol levels between the groups which might explain differing times to glycemic control. Intravenous reductions with feeding increments were also standardized for the study to ensure comparable treatment strategies aside from the provided diazoxide or placebo.



